April 2013 Critical Care Journal Club
 Friday, April 26, 2013 at 3:50PM
Friday, April 26, 2013 at 3:50PM We welcomed intensivists from Banner Health to video-conference with us as we discussed several articles, and evaluated the ACP Journal Club – another good resource for keeping up to date.
Hill NS. Review: Lower rather than higher tidal volume benefits ventilated patients without ARDS. Ann Intern Med. 2013;158:JC4. Abstract
Lauzier F. Hydroxyethyl starch 130/0.4 and saline did not differ for mortality at 90 days in ICU patients. Ann Intern Med. 2013;158:JC5. Abstract
The April ACP Journal Club reviewed two critical care articles – a meta-analysis that concluded that low tidal volume ventilation reduced mortality in patients without ARDS, and a large RCT that showed no mortality difference between critically-ill patients resuscitated with hydroxyethyl starch versus saline. Both articles were awarded 6/7 stars for “clinical impact”, yet neither article had any impact on our clinical practice. This troubled us.
We could think of 4 necessary criteria in order for research to have legitimate clinical impact: 1) internal validity; 2) external applicability; 3) an intervention that is different from what we are already doing; and 4) a clinically significant benefit. We had previously critically appraised the study on low tidal volume ventilation in non-ARDS patients, found it sorely lacking in internal validity, therefore rejecting the practice for clinical application. Two-thirds of the patients included in this meta-analysis were enrolled in studies that were only observational in design. Many of the included studies focused on short duration, post-op mechanical ventilation. It seemed biologically implausible to us that alveolar hyperinflation could lead to ventilator-associated lung injury in patients intubated for a few days with essentially normal post-op lungs. The ACP reviewer endorsed low tidal volume ventilation in non-ARDS patients in part because of the “lack of evidence to suggest harm”. Yet low tidal ventilation can be uncomfortable for an awake patient, and can only be maintained in some by increasing sedation medications. There is good evidence that this can lead to prolonged ventilator LOS and associated complications. Observational studies lumped into a meta-analysis should only be used to generate hypotheses. Therefore, we would have rated this article as 0/7 stars for clinical impact - no change warranted in clinical practice, but further study recommended.
The second critical care study in the ACP journal club has high internal validity, but did not change our practice either, because we already knew from multiple previous studies that colloid resuscitation fluids were not superior to crystalloids, and increase the risk of acute kidney injury in critically-ill patients. We would have rated this article as 1/7 stars for clinical impact because it might change the practice of a minority of clinicians who are still routinely using colloid resuscitation, if the previous studies hadn’t already convinced them to do so.
It is our observation that the reviewers for ACP journal club generally overestimate the clinical impact of the studies they review. We recommend that they should develop a precise and reasonable definition of “clinical impact” such as we have proposed, and use it to provide more accurate critical appraisal ratings.
Ioannidis JP. Are mortality differences detected by administrative data reliable and actionable? JAMA. 2013;309:1410-1. Abstract
We have frequently reviewed articles that use DRG billing codes databases. The convenience and large size of these databases makes them tempting to analyze, but it has been repeatedly demonstrated that they are often not accurate for research purposes. Financial incentives have been shown to significantly alter hospital coding procedures resulting in over or under-coding of certain diagnoses, comorbidities and complications (depending on the incentives). Billing codes that don’t accurately reflect the patient’s true clinical condition can lead to errors in identifying patients with the diagnosis of interest, adjusting for severity of illness, and determining outcomes and interventions might have affected the outcome. Administrative database studies may also be overpowered. This might seem like a good thing, but very large sample sizes can result in “statistically significant” differences that are clinically infinitesimally small. We feel that observational studies based on administrative databases should be viewed as hypothesis-generating. In almost all cases, their internal validity is inferior to observational studies based on clinical data.
Weber-Carstens S, Schneider J, Wollersheim T, Assmann A, Bierbrauer J, Marg A, Al Hasani H, Chadt A, Wenzel K, Koch S, Fielitz J, Kleber C, Faust K, Mai K, Spies CD, Luft FC, Boschmann M, Spranger J, Spuler S. Critical illness myopathy and GLUT4: significance of insulin and muscle contraction. Am J Respir Crit Care Med. 2013;187:387-96. Abstract
This study sought to clarify the mechanism of critical illness myopathy, and test the concept of whether electrically-induced muscle stimulation might prevent it. It was not meant to immediately impact clinical care, but to guide further research. I appreciate Emad volunteering to review it, as it is very intimidating reading. I’m going to condense our take on it.
The authors screened 874 critically ill patients in order to complete a study of only 35. Thirty of these underwent electrophysiological testing with an insulin clamp, of which 12 had critical illness myopathy (CIM), and 18 did not. The other five patients underwent electrical stimulation of a unilateral vastus lateralis muscle in an attempt to prevent CIM, using the contralateral unstimulated muscle as a control.
Normally, glucose uptake in muscle cells is facilitated by glucose transporter 4 (GLU4) – which is stored in perinuclear vesicles, and rapidly released and translocated to the sarcolemma in response to insulin receptor binding, or muscle contraction. However, it was found that this mechanism fails in critical illness. Muscle glucose utilization was not stimulated by high-dose insulin in study subjects. Insulin resistance was demonstrated in all the critically-ill patients, but was worse in those with CIM, in whom immunohistochemical studies of muscle tissue showed that GLU4 was essentially trapped in the perinuclear vesicles. Several signal transduction proteins that regulate GLU4 were shown to be significantly under-expressed in these patients – perhaps explaining why this might be.
In the patients who underwent periodic electrical muscle stimulation during their illness, these transduction proteins were restored, GLU4 release into the sarcolemma was maintained, and the muscle fibers showed robust morphological improvements compared to the unstimulated contralateral leg muscles. This study helps expand our understanding of the pathogenesis of critical illness myopathy and insulin resistance, and suggests a method to prevent CIM that deserves further clinical study.
Prasad V, Rho J, Cifu A. The inferior vena cava filter: how could a medical device be so well accepted without any evidence of efficacy? JAMA Intern Med. 2013;173:493-5. Abstract
Although the inferior vena cava (IVC) filter is FDA-approved, and use has steadily climbed to over 50,000 device placements per year, there is no valid evidence of clinical efficacy. The study that initially supported use of IVC filters was an uncontrolled case series reported by Greenfield. He observed a 4% rate of pulmonary embolism (PE) after IVC filter placement, but nearly half the patients were lost to follow-up. The true rate of PE in patients with Greenfield filters is unknowable in such a study, because it’s possible that patients who suffered PE were more likely to be lost to follow-up. A single randomized controlled trial (RCT) addresses the clinical efficacy of IVC filters. Four-hundred patients with venous thromboembolism were randomized to receive IVC filters in addition to antithrombotic therapy. Interestingly, patients who had the most widely accepted indication of IVC filters – those with contraindications to antithrombotic therapy - were excluded from this study. At a two-year follow-up, there was no significant reduction in PE or mortality in the group with IVC filters, but a statistically significant absolute increase in the rate of deep venous thrombosis by 10%. At an eight-year follow-up, the rate of PE as elicited by an annual phone interview was statistically significantly reduced in patients with an IVC filter. The rate of DVT remained significantly higher in patients with filters, and mortality remained unaffected.
This RCT has little clinical relevance since IVC filters are not usually recommended for the type of patients enrolled in the study. Nor are IVC filters placed in order to prevent pulmonary embolism occurring more than 2 years later. The study did show convincingly that IVC filters increase the rate of DVT. Other studies have shown complications including fracture and fragment embolization, and whole filter embolization which have in some cases caused cardiac arrest and fatality. One study showed a 33% risk for IVC occlusion at 9 years. Retrievable filters seemed to have been widely clinically accepted, but retrieving them would mitigate the only possibly proven benefit (preventing PE occurring years later) – in any case, another study suggests that as few as 10% of retrievable filters are ever retrieved.
The authors challenge the lack of support for FDA approval of these devices, and point out that there is no financial incentive for further research by the manufacturers, so long as physicians are willing to use them without evidence of efficacy.
A spirited discussion expressed a wide spectrum of views. We discussed the common use of prophylactic IVC filters in patients with a high risk for primary or recurrent venous thromboembolism – for instance in trauma or cancer patients. Some felt that the standard of care demands use of IVC filters in this situation, even in the absence of scientific evidence of benefit. Others felt that IVC filters are highly over-utilized in these situations. In light of our earlier discussion, this is an article that I feel has legitimate clinical impact. Many of us will still probably consider IVC filters for patients with acute venous thromboembolism who have strong contraindications to antithrombotic therapy, despite the absence of evidence that it will prevent immediate life-threatening pulmonary embolism. But this article will likely make us more cautious in recommending this potentially dangerous device to patients with other proposed indications.
In addition to inducing DVT, IVC filters can induce propagation of the clot. If there are contraindications to anticoagulation, this might induce the placement of additional filters, further propagation of the clot, additional filters, etc. (Figure 1).
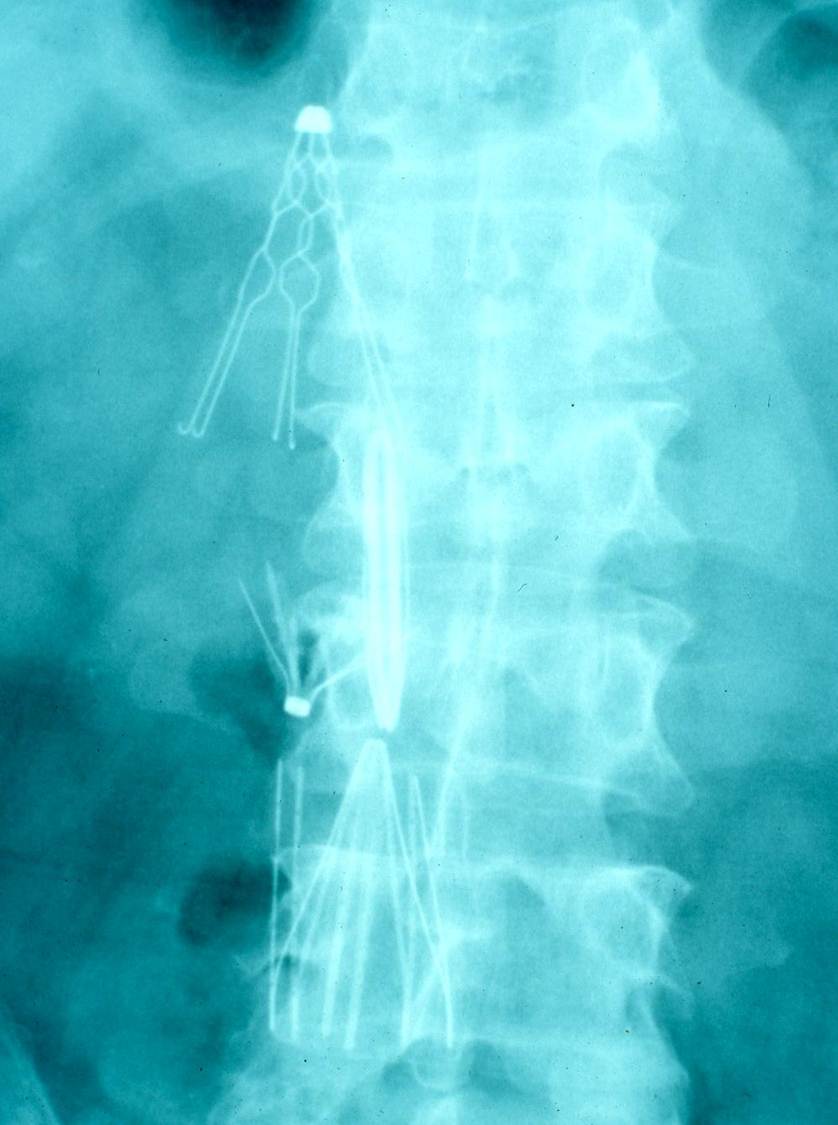
Figure 1. Patient with multiple IVC filters placed.
I was fascinated by our group reaction to this article. It is apparent that most of us still struggle when our logic conflicts with empirical evidence. In 1984, Goodwin coined the term “tomato effect” to describe the situation in which an efficacious treatment for a certain disease is ignored or rejected because it doesn’t make sense in light of accepted theories of disease mechanisms. [The term was chosen because a widely-held misconception in North American in the 1600 and 1700’s that tomatoes were poisonous – causing consumption of tomatoes to lag far behind their acceptance in Europe]. Goodwin contrasted the tomato effect with the placebo effect. But in the case of IVC filters, I’m not sure the term placebo effect is applicable. Many physicians are so convinced that IVC filters ought to work, that they are willing to forego scientific proof that they do work. Maybe we should call this the “Swan effect” since for years we placed Swan-Ganz pulmonary artery catheters before evidence was published that they provide little or no benefit to the patient.
Robert A. Raschke MD, MS
Associate Editor
Reference as: Raschke RA. April 2013 critical care journal club. Southwest J Pulm Crit Care. 2013;6(4):191-5. PDF

