Evan Denis Schmitz MD
La Jolla, CA USA
Abstract
A case of severe respiratory disease associated with vaping cannabinoid oil is reported in a 38-year-old woman. She presented with shortness of breath and nonproductive cough. Chest x-ray and CT scan showed diffuse ground glass opacities and consolidation. Bronchoscopy showed diffuse bronchial erythema and bronchoalveolar lavage contained an increased percentage of eosinophils (59%). She was treated with high dose corticosteroids and rapidly improved.
Case Report
History of Present Illness
A 38-year-old woman complained of worsening shortness of breath and nonproductive cough for four weeks. She used to be able to climb three flights of stairs but now can barely walk ten feet. She had been treated with various forms of antibiotics, inhalers and steroids and was taking 20 mg of prednisone a day on the day of hospitalization. She also received opiates to help control her cough. She denied any hemoptysis, fever, chills, or sputum production. Because of her progressive symptoms she was hospitalized for further evaluation and management.
Past Medical History, Social History and Family History
She has a history of obesity and fibromyalgia. She has a prior history of smoking one to two packs a day for five years quitting approximately 15 years ago. Because of a family crisis she tried vaping cannabidiol (CBD) oil approximately one month prior to admission. She also resumed smoking tobacco one half a pack per day. Her family history was unremarkable.
Medications
She was taking prednisone 20 mg/day and cyclobenzaprine (Flexeril®) for her fibromyalgia. She was also taking codeine cough syrup.
Review of Symptoms
She did have some chest pain associated with her shortness of breath as well as chronic muscle aches and intermittent lower extremity edema. Her review of systems was otherwise unremarkable.
Physical Examination
Vital Signs: BP 137/72 mm Hg, Pulse 84 beats/min, temperature 98.8 °F, respirations 22 breaths/min, height 5’0, weight 231 lbs, SpO2 96%
General: She was morbidly obese and only able to speak in short sentences.
Mouth: Moist. Mallampati 3.
Pulmonary: Faint expiratory crackles. No wheezing.
Cardiovascular: Normal rate, regular rhythm, normal heart sounds and intact distal pulses. Exam reveals no gallop and no friction rub. No murmur heard.
Abdominal: Soft, bowel sounds normal. No distension, mass or tenderness. No rebound or guarding. Centripetal obesity.
Extremities: Normal range of motion. No edema or tenderness.
Lymphatics: No cervical or supraclavicular adenopathy.
Neurological: Alert and oriented to person, place and time.
Skin: Warm and dry. No rash, erythema or pallor. Not diaphoretic. Capillary refill within normal limits. No skin tenting.
Psychiatric: Depressed mood.
Laboratory
Pertinent findings are on her laboratory evaluation include an elevated white blood cell count of 16,850 cells/µL with an increased number of neutrophils. Her electrolytes, liver enzymes, creatinine, blood urea nitrogen and urinalysis were within normal limits.
Radiology
Her admission chest x-ray is shown in Figure 1.
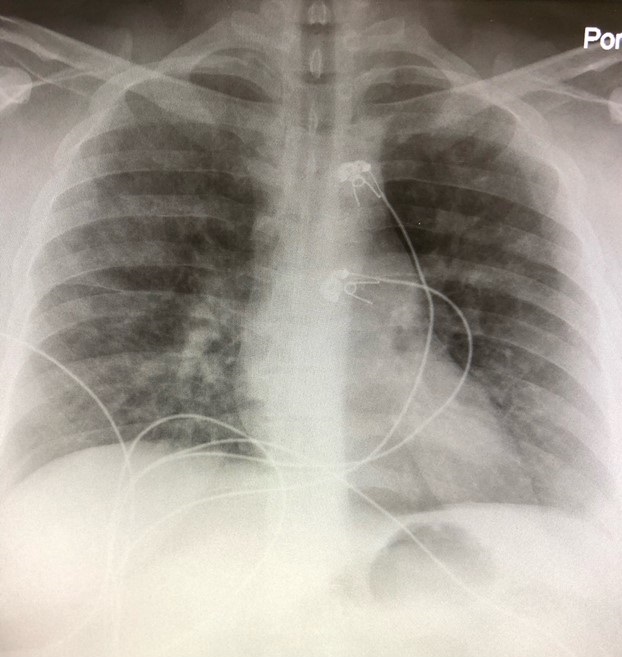
Figure 1. The admission portable chest x-ray showed bilateral patchy pulmonary infiltrates.
To better define the areas of consolidation, a thoracic CT scan was performed (Figure 2).
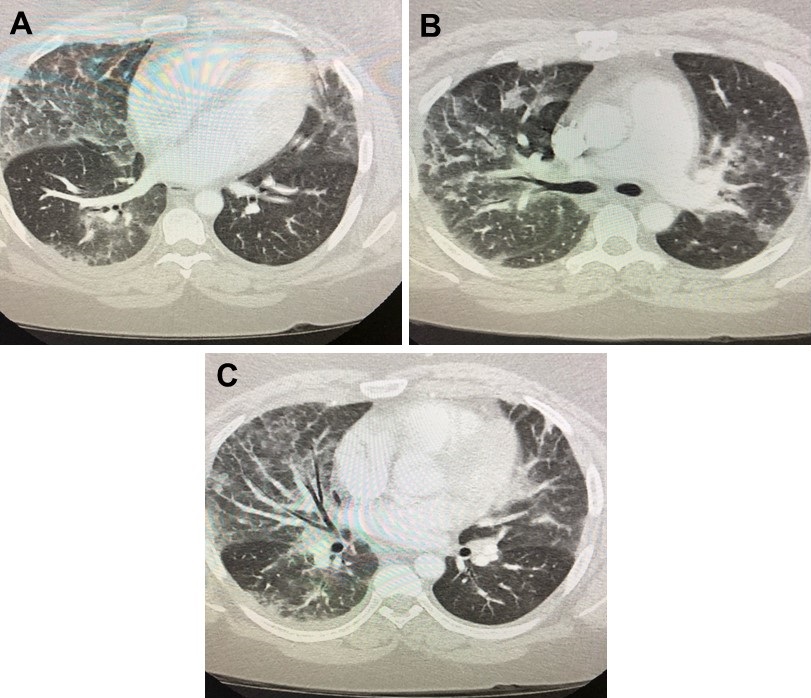
Figure 2. Representative images in lung windows from contrast enhanced thoracic CT scan showing nonspecific patchy areas of ground glass and alveolar opacities with septal thickening involving both lungs.
Hospital Course
Echocardiography was unremarkable. Bronchoscopy with bronchoalveolar lavage was performed. She had diffuse upper and lower airway erythema and considerable coughing during the procedure. The cell differential revealed an increase in eosinophils (59%) and multiple foamy macrophages. Smears and cultures of the lavage fluid were negative for pathogens. She was treated with high dose corticosteroids (methylprednisolone 1000 mg/day). She rapidly improved over four days with her cough and shortness of breath resolving. A chest x-ray at discharge revealed improvement of the pulmonary infiltrates (Figure 3).
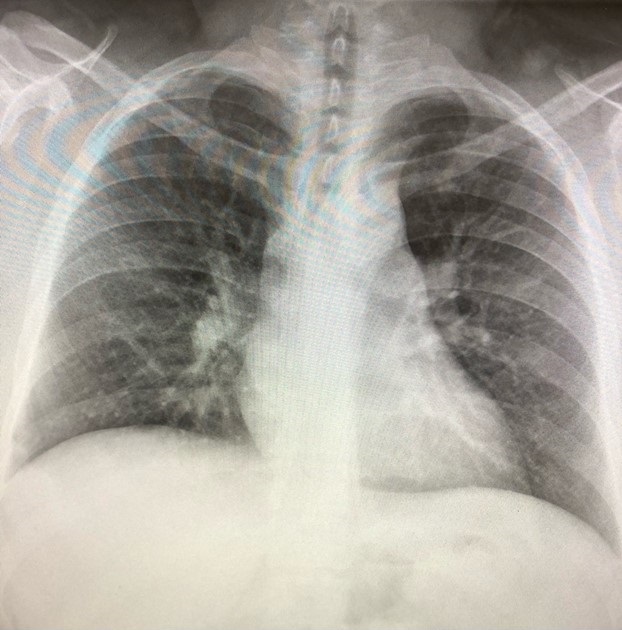
Figure 3. Chest x-ray on the morning of discharge showing near resolution of her pulmonary infiltrates.
Discussion
At the time of this writing (9/21/19) there have been 530 cases of lung injury associated with e-cigarette product use or vaping reported with seven deaths (1). Nearly three fourths (72%) of cases have been male with two thirds (67%) 18 to 34 years old. Most patients have reported a history of using e-cigarette products containing tetrahydrocannabinol (THC). Many patients have reported using THC and nicotine. Some have reported the use of e-cigarette products containing only nicotine.
At present no specific e-cigarette or vaping product (devices, liquids, refill pods, and/or cartridges) or substance has been linked to all cases. It seems likely that there may be different mechanisms of lung injury from different substances. In support of this concept, the present case had high numbers of eosinophils in the bronchoalveolar lavage while other cases have shown an increase in neutrophils (2). Our patient was treated with high dose corticosteroids and did improve while on the corticosteroids. However, the time course does not establish a definite relationship between corticosteroid treatment and her improvement.
At present the CDC recommends refraining from using e-cigarette or vaping products (1). Anyone who uses an e-cigarette or vaping product should not buy these products (e.g., e-cigarette or vaping products with THC or CBD oils) off the street, and should not modify or add any substances to these products that are not intended by the manufacturer.
References
- CDC. Outbreak of lung injury associated with e-cigarette use, or vaping. September 19, 2019. Available at: https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html (accessed 9/21/19).
- Arizona Thoracic Society. September 2019 Arizona thoracic society notes. Southwest J Pulm Crit Care. 2019;19(3):99-100. [CrossRef]
Cite as: Schmitz ED. Severe respiratory disease associated with vaping: a case report. Southwest J Pulm Crit Care. 2019;19(3):105-9. doi: https://doi.org/10.13175/swjpcc062-19 PDF