Oscar Furet, RN M.P.H.
Arizona Arthritis Center, University of Arizona, Tucson, AZ
oscarf@arthritis.arizona.edu
James L. Goodwin, Ph.D.
Arizona Respiratory Center, University of Arizona, Tucson, AZ
jgoodwin@email.arizona.edu
Stuart F. Quan, M.D.
Arizona Respiratory Center, University of Arizona, Tucson, AZ. Division of Sleep Medicine, Brigham and Womens Hospital and Harvard Medical School, Boston, MA
Correspondent: Stuart F. Quan, M.D.
Division of Sleep Medicine
Harvard Medical School
401 Park Dr., 2nd Floor East
Boston, MA 02215
Email: squan@arc.arizona.edu
Voice: 617-998-8842
Fax: 617-998-8823
Reference as: Furet O, Goodwin JL, Quan SF. Incidence and remission of parasomnias among adolescent children in the Tucson Children’s Assessment of Sleep Apnea (TuCASA) Study. Southwest J Pulm Crit Care 2011;2:93-101. (Click here for PDF version)
Abstract
Background: Longitudinal assessments of parasomnias in the adolescent population are scarce. This analysis aims to identify the incidence and remission of parasomnias in the adolescent age group.
Methods: The TuCASA study is a prospective cohort study that initially enrolled children between the ages of 6 and 11 years (Time 1) and subsequently re-studied them approximately 5 years later (Time 2). At both time points parents were asked to complete a comprehensive sleep habits questionnaire designed to assess the severity of sleep-related symptoms that included questions about enuresis (EN), sleep terrors (TR), sleep walking (SW) and sleep talking (ST).
Results: There were 350 children participating at Time 1 who were studied as adolescents at time 2. The mean interval between measurements was (4.6 years). The incidence of EN, TR, ST, and SW in these 10-18 year old children was 0.3%, 0.6%, 6.0% and 1.1% respectively. Remission rates were 70.8%, 100%, 64.8% and 50.0% respectively.
Conclusions: The incidence rates of EN, TR, and SW were relatively low moving from childhood to adolescence while remission rates were high across all parasomnias.

Introduction
Parasomnias are unpleasant or undesirable behavioral or experiential phenomena which occur predominantly or exclusively during sleep.1 When occurring during childhood, they can result in substantial parental sleep disruption, anxiety and concern. In addition, there may be adverse consequences on the child's behavior and self-esteem.2-4 There are 4 parasomnias that are commonly observed during childhood. Sleepwalking (SW) and sleep terrors (NT) are parasomnias associated with arousal that usually occur during slow wave sleep.5 Sleepwalking is semi-purposeful ambulatory behavior without awareness. Night terrors (also called sleep terrors) are recurrent episodes of abrupt awakening from deep non-REM sleep, usually with a scream and signs of intense fear and autonomic arousal.5 Sleep talking (somniloquy) (ST) consists of vocalizations, frequently nonsensical, during both REM and non-REM sleep.6 Enuresis (EN) is characterized by recurrent involuntary micturition that occurs during sleep.7 In contrast to SW, and NT, enuresis may occur during non-rapid eye movement (NREM) or rapid eye movement (REM) sleep.8
Epidemiological surveys investigating parasomnias in the general population are uncommon, perhaps because these parasomnias are usually considered harmless childhood occurrences. However, the prevalence of parasomnias in the general population of children has been estimated at approximately 3%–17% for SW,5, 9, 10 1%–7% for NT,5, 9 2%–18% for EN9-12 and 5%–27% for ST. 9-12 These estimates vary greatly because rarely are the same definitions for the frequency of events used. Although it is generally accepted that childhood parasomnias remit with age, virtually all studies have been cross-sectional. 4, 10, 11, 13-15 To our knowledge, there have been no studies investigating the remission and incidence of parasomnias in a community-based adolescent population. Therefore, it is the purpose of this analysis to describe the incidence and remission of parasomnias in such a cohort using data from the Tucson Children’s Assessment of Sleep Apnea (TuCASA) study.
Methods
TuCASA was designed to investigate the incidence, prevalence and correlates of objectively measured sleep-related breathing disorders (SRBD) in a prospective cohort study of preadolescent Hispanic and Caucasian children ages 6 to 12 years. Detailed recruitment methods have been described previously.16 Briefly, Hispanic and Caucasian children ages 6 to 12 years were recruited through the Tucson Unified School District (TUSD), a very large district with a substantial elementary school population. Parents were asked to complete a short screening questionnaire and to provide their contact information if they were willing to allow study personnel to contact them to determine if their child was eligible for the study. A total of 7,055 screening questionnaires were sent home with children in a “notes home” folder. Of these, 2,327 (33%) were returned. Recruitment information was supplied on 52% of the returned questionnaires. From these questionnaires, children were selected for potential participation based on pre-established inclusion and exclusion criteria, and after parents gave informed consent and the child gave assent using language-appropriate IRB approved forms. The TuCASA protocol was approved by both the University of Arizona Human Subjects Committee and the TUSD Research Committee.
Initially from 1999-2003, 503 children were enrolled (Time 1) and subsequently 350 were re-studied approximately 5 years later (Time 2). In addition to undergoing home polysomnography at both time points, parents were asked to complete a comprehensive sleep habits questionnaire (SHQ) that recorded the characteristics of their child’s sleep history including questions about EN, NT, SW and ST.
Specific questions were the following: "Does this child sleepwalk?", and "Does this child talk in his or her sleep? (Talk without being fully awake?)". For these 2 questions, possible responses were "Never", "less than three times per month", "three to five times per month", or "more than five times per month". The occurrence of these parasomnias was defined as follows: SW was present if it was reported more than three times per month, and ST was present if it was reported more than five times per month. Additionally, the parent was asked "How often does this child awaken at night afraid or appearing tearful?” If the parent answered that the child had more than five fearful awakenings per month then the child was classified as having NT. EN was present if it was reported as occurring more than five times per month. These definitions were chosen to be consistent with our previous analyses of parasomnias in this cohort and were thought to be clinically meaningful when these children were preadolecents.9
The SHQ was also used to define the occurrence of habitual snoring (SN), excessive daytime sleepiness (EDS), witnessed apnea (WITAP), difficulty initiating and maintaining sleep (INSOM), and learning problems (LP). These sleep problems were considered present if they were reported 'frequently' or more (5 or more times per week). Although the specific range and order of questions used on the TuCASA SHQ and screening questionnaires have not been previously validated, key questions in the questionnaire have face validity and were taken from those used by Carroll and colleagues.17
As described in previous analyses from the TuCASA cohort,9,16 we computed a respiratory disturbance index (RDI) as the total number of apneas and hypopneas/total sleep time (TST). Hypopneas were required to have an associated oxygen desaturation of 3%. Sleep disordered breathing (SDB) was considered present if the RDI was > 1 event/hour TST.
Statistical analysis of the data was performed using Stata 10 (StataCorp LP, College Station, TX) and IBM SPSS Statistics 18 (New York, NY). As appropriate, comparisons of means and proportions were performed using two-sample t-tests for continuous data, and chi-squared tests and the exact binomial test for categorical data. Data are expressed as means + SD and percentages.
Results
There were a total of 503 children participating at Time 1 and 350 adolescents at time 2. Characteristics for the study group are shown in Table 1.
Table 1: Description of the TuCASA Cohort at Time 1 and Time 2
|
|
Time 1
|
Time 2
|
p
|
|
Number in Cohort
|
503
|
350
|
-
|
|
Age (Mean + SD)
|
8.8 ± 1.6
|
13.3 ±1.7
|
-
|
|
Age (Min-Max)
|
[6,12.6]
|
[9.9,17.5]
|
-
|
|
Gender (% Male)
|
50
|
51
|
0.7643
|
|
Ethnicity (% Caucasian)
|
58
|
63
|
0.1283
|
|
Standardized BMI (Mean + SD)
|
.30 ± 1.2
|
.50 ± 1.1
|
0.0249
|
|
Obesity (%)
|
13
|
24
|
0.017
|
The mean age at first assessment was 8.8 years (min/max: 6-12.6 years) while mean age at second assessment was 13.3 years (min-max: 9.9-17.5 years). The mean time between assessments was 4.6 years (range: 2.9-7.3 years). There were 51% males and 49% females at the Time 2, approximately the same ratio as Time1. The gender ratio remained approximately the same at both measurements. Notably, standardized BMI increased and the % of the cohort classified as obese increased over the time interval. However, standardized BMI was not significantly higher in those children with parasomnias (data not shown).
As shown in Table 2, at Time 1 there were no differences in the prevalence rates of all 4 parasomnias using the entire cohort in comparison to a cohort restricted only to those children who had assessments made at both time points (Restricted Cohort). In addition, the prevalence of parasomnias at Time 2 remained similar to the prevalence at Time 1 with the exception of EN which declined markedly from 7% to 2%. Also shown in Table 2 are the prevalence, remission and incidence rates of the 4 parasomnias at Time 2. The incidence of EN, TR, and SW in our 10-17 year old children were approximately 1% for all 3 contrasting with ST with an incidence rate of approximately 6%. Furthermore, remission rates were high for all the parasomnias. All 9 adolescents had remission from NT. 17 of 24 subjects, approximately 71%, had remission from EN. 24 of 37 participants had remission from ST, approximately 65%. 1 out of 2 subjects (50%) with SW had remission. Incidence and remission of all parasomnias were not related to SN, WITAP, INSOM, or LP although limited incidence and remission numbers precluded extensive meaningful analyses. At Time 1, the prevalence of SDB was 27.8% (89/320). At Time 2, 14.4% (46/320) Children with SDB on both occasions were 25/320 or 7.8%. There were 15 boys and 10 girls with persistent SDB with no ethnic differences. Because of the relatively small numbers of children with parasomnias and SDB at Time 2, we were unable to determine whether persistent SDB was a risk for prevalent or incident parasomnias .

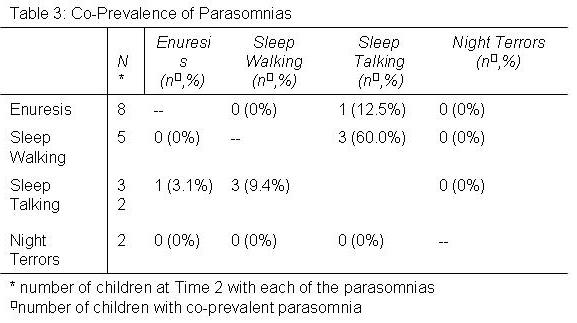
In Table 3 is shown the number and percent of parasomnias that occurred in association with other parasomnias at Time 2. Except for an association between sleep walking and sleep talking, parasomnias occurred independent of each other.
Discussion
The TuCASA study has documented the prevalence, incidence and remission of parasomnias in a population-based sample of 10-17 year old subjects. We found that incidence rates for parasomnias were very low for EN NT and SW and remission rates were high for all parasomnias. Furthermore, incidence and remission rates did not appear to be related to symptoms of sleep disturbances or learning problems, and except for sleep walking and sleep talking, they generally were not co-prevalent.
In this study, except for ST, prevalence rates for parasomnias in our cohort of adolescents were relatively low. Available data documenting the prevalence rates of various parasomnias in this age group are relatively sparse with previous reports largely restricted to preadolescents. 4, 9-12, 15 However, with respect to SW, 3 previous studies in adolescents have observed prevalence rates ranging from 3 to 15% which are higher than the 1.4% noted in our study.18-20 Inconsistent prevalence rates have been noted for NT with one study reporting rates <4%,20 but another reporting 10.2%.19 In contrast, the prevalence of enuresis 7, 18, 20 and the prevalence of ST 6,13, 20 in adolescents have been reported to be consistently low and high respectively. Our data are concordant with these previous reports. However, the relevance of these comparisons is unclear since no standard method of assessing the frequency of parasomnias exists. Our requirement that these events occurred more than three to five times per month are more stringent than those employed in most studies. Thus, it not surprising that our prevalence rates for SW and NT in adolescence are discordant with previous observations.
There is general consensus that childhood parasomnias remit as children develop from childhood to adolescence and that few adolescents develop them.5, 21 However, this impression is based primarily on empiric observations because there are few longitudinal studies.20, 22, 23 The largest longitudinal study prospectively interrogated parents of children at age 10 through age 13 years, but retrospectively questioned parents to determine if a parasomnia was present between ages 3 and 9 years.20 In this study, prevalence rates were noted to decline markedly by age 13 years to 3.3%, 1.2% and 2.0% for SW, NT and EN, respectively. In contrast, there was only a slight nonsignificant decrease in ST with 29.2% of children still having this condition at age 13 years. Our data are generally consistent with the results of this previous study although our prevalence of ST is somewhat lower. However, we extend these foregoing findings by documenting incidence and remission rates. Except for ST, very few adolescents developed new parasomnias. Nonetheless, although remission rates were high, some participants in this study had persistent symptoms which likely continue through into adulthood.24, 25
We previously examined the prevalence of parasomnias in the TuCASA cohort when the children were preadolescents.9 We found an associations between parasomnias, and SDB, symptoms of other sleep disturbances and learning problems. Unfortunately, because of the small numbers of children with parasomnias in this follow-up cohort, we were unable to determine whether these latter findings are still present.
In this study, standardized BMI increased as did the % of the cohort classified as obese. While these observations most likely are a reflection of the ongoing obesity epidemic in the United States, those with parasomnias did not have significantly higher standardized BMI.
Some evidence indicates that individuals with one parasomnia have a greater likelihood of having another one.20, 25 Consistent with these previous studies, we found a modest association between ST and SW, both of which are disorders of arousal. Otherwise, we found little evidence to support the contention that parasomnias are more likely to be co-prevalent.
Although our study is the first to prospectively document the incidence and remission of parasomnias in a large general population of children, it is not without some limitations. First, it is possible that parents underestimated the actual number of parasomnias that occurred. Depending on the bedtime of the child and the severity of the event, it is probable that parents are not awake during every occurrence. Second, recruitment may have incurred a selection bias so that parents who agreed to have their children participate might be more likely to have symptomatic children than those who did not. We think this unlikely because the focus of the study was SDB and it is unlikely that parents agreed to have their children participate based on the presence of parasomnias. Lastly, there were 153 subjects that participated at Time 1 but that did not participate at Time 2. There was no difference related to gender between those that participated at Time 2 and those that did not although slightly more Hispanic adolescents were lost to follow-up than Caucasians. In addition, the prevalence rates of parasomnias in the entire cohort and the restricted were the same. Thus, we do not believe that the restricted cohort of children who had data at both time points was markedly different than the larger group of children recruited at Time 1.
In conclusion, although parasomnias are relatively common in childhood, our study demonstrates that most remit, and that the development of parasomnias in older children is uncommon. Our findings provide objective data supporting the generally accepted perception that most parasomnias in children will resolve over time.
Acknowledgements
This study was supported by Grant HL 62373 from the National Heart Lung and Blood Institute. All authors contributed to the writing and analyses contained in the study and had full access to the data. Dr. Quan is the Principal Investigator of the TuCASA study. Drs. Quan and Goodwin supervised the recruitment of participants and the operations of TuCASA.
References
1 . Matwiyoff G, Lee-Chiong T. Parasomnias: an overview. Indian J Med Res 2010; 131:333-337.
2 . Heussler HS. 9 Common causes of sleep disruption and daytime sleepiness: childhood sleep disorders II. Med J Aust 2005; 182(9):484-489.
3 . Chokroverty S. Overview of sleep & sleep disorders. Indian J Med Res 2010; 131:126-140.
4 . Agargun MY, Cilli AS, Sener S, et al. The prevalence of parasomnias in preadolescent school-aged children: a Turkish sample. Sleep 2004; 27(4):701-705.
5 . Avidan AY, Kaplish N. The parasomnias: epidemiology, clinical features, and diagnostic approach. Clin Chest Med 2010; 31(2):353-370.
6 . Arkin AM. Sleep-talking: a review. J Nerv Ment Dis 1966; 143(2):101-122.
7 . Hansakunachai T, Ruangdaraganon N, Udomsubpayakul U, Sombuntham T, Kotchabhakdi N. Epidemiology of enuresis among school-age children in Thailand. J Dev Behav Pediatr 2005; 26(5):356-360.
8 . Mikkelsen EJ, Rapoport JL, Nee L, Gruenau C, Mendelson W, Gillin JC. Childhood enuresis. I. Sleep patterns and psychopathology. Arch Gen Psychiatry 1980; 37(10):1139-1144.
9 . Goodwin JL, Kaemingk KL, Fregosi RF, et al. Parasomnias and sleep disordered breathing in Caucasian and Hispanic children - the Tucson children's assessment of sleep apnea study. BMC Med 2004; 2:14.
10 . Arruda MA, Guidetti V, Galli F, Albuquerque RC, Bigal ME. Childhood periodic syndromes: a population-based study. Pediatr Neurol 2010; 43(6):420-424.
11 . Liu X, Ma Y, Wang Y, et al. Brief report: An epidemiologic survey of the prevalence of sleep disorders among children 2 to 12 years old in Beijing, China. Pediatrics 2005; 115(1 Suppl):266-268.
12 . Simola P, Niskakangas M, Liukkonen K, et al. Sleep problems and daytime tiredness in Finnish preschool-aged children-a community survey. Child Care Health Dev 2010; 36(6):805-811.
13 . Garcia-Jimenez MA, Salcedo-Aguilar F, Rodriguez-Almonacid FM, et al. The prevalence of sleep disorders among adolescents in Cuenca, Spain. Rev Neurol 2004; 39(1):18-24.
14 . Ipsiroglu OS, Fatemi A, Werner I, Tiefenthaler M, Urschitz MS, Schwarz B. Prevalence of sleep disorders in school children between 11 and 15 years of age. Wien Klin Wochenschr 2001; 113(7-8):235-244.
15 . Petit D, Touchette E, Tremblay RE, Boivin M, Montplaisir J. Dyssomnias and parasomnias in early childhood. Pediatrics 2007; 119(5):e1016-25.
16 . Goodwin JL, Enright PL, Kaemingk KL, et al. Feasibility of using unattended polysomnography in children for research--report of the Tucson Children's Assessment of Sleep Apnea study (TuCASA). Sleep 2001; 24(8):937-944.
17 . Carroll JL, McColley SA, Marcus CL, Curtis S, Loughlin GM. Inability of clinical history to distinguish primary snoring from obstructive sleep apnea syndrome in children. Chest 1995; 108(3):610-618.
18 . Klackenberg G. Incidence of parasomnias in children in a general population. In: Sleep and its Disorders in Children. Guilleminault C, ed. New York: Raven Press, 1987; 99-113
19 . Ipsiroglu OS, Fatemi A, Werner I, Paditz E, Schwarz B. Self-reported organic and nonorganic sleep problems in schoolchildren aged 11 to 15 years in Vienna. J Adolesc Health 2002; 31(5):436-442.
20 . Laberge L, Tremblay RE, Vitaro F, Montplaisir J. Development of parasomnias from childhood to early adolescence. Pediatrics 2000; 106(1 Pt 1):67-74.
21 . Guilleminault G. Disorders of arousal in children: Somnambulism and night terros. In: Guilleminault C, ed. New York: Raven Press, 1987; 243-252
22 . DiMario FJ,Jr, Emery ES,3rd. The natural history of night terrors. Clin Pediatr (Phila) 1987; 26(10):505-511.
23 . Klackenberg G. Somnambulism in childhood--prevalence, course and behavioral correlations. A prospective longitudinal study (6-16 years). Acta Paediatr Scand 1982; 71(3):495-499.
24 . Oluwole OS. Lifetime prevalence and incidence of parasomnias in a population of young adult Nigerians. J Neurol 2010; 257(7):1141-1147.
25 . Bjorvatn B, Gronli J, Pallesen S. Prevalence of different parasomnias in the general population. Sleep Med 2010; 11(10):1031-1034.
 Wednesday, September 5, 2012 at 9:26AM
Wednesday, September 5, 2012 at 9:26AM