Fluid in the Management of the Acute Respiratory Distress Syndrome
 Wednesday, June 19, 2013 at 3:02PM
Wednesday, June 19, 2013 at 3:02PM Sanjaya Karki* (drsanjaya.karki@yahoo.com)
Yong-Jie Yin* (corresponding author)-yongjieyin2003@yahoo.com.cn
Jing-Xiao Zhang*
Nijamudin Samani*
Dipesh Pradhan‡
Sangeeta Singh Deuja (Karki)†
Reshma Karki#
Raghvendra Thakur**
Nan Zhao***
*Department of Emergency and Critical Care Medicine, Second Hospital of Jilin University, Changchun, China
‡ First hospital of Jilin University, China
†University of Huddersfield, UK
#Sri Birendra Hospital, Nepal
**Second Hospital of Jilin University
***Department of Chemistry, Jilin University, Changchun, China
Abstract
Introduction
Non-cardiogenic pulmonary edema is the hallmark of the acute respiratory distress syndrome (ARDS). The amount of fluid and which fluid should be used in these patients is controversial.
Methods
43 patients with ARDS treated in the intensive care unit (ICU) of the Second Hospital, Jilin University between November 1, 2011-November 1, 2012 were prospectively analyzed and was observational. Volume and the type of fluid administered were compared to 90 day mortality and the 24 and 72 hour sequential organ failure assessment (SOFA) score, lactate level, oxygenation index (PaO2/FiO2), duration of ICU stay, total ventilator days, and need for continuous renal replacement therapy (CRRT).
Results
Mortality was increased when hydroxylethyl starch (HES) was used in the first day or plasma substitutes were used during the first 3 days (P<0.05, both comparisons). Volumes of fluid >3000 ml during the first 24 hours or >8000 ml during the first 72 hours were associated with higher SOFA scores at 24 and 72 hours (P<0.05, both comparisons). Colloid, especially higher volume colloid use was also associated with increased SOFA scores at either 24 or 72 hours.
Conclusions
Limiting the use of colloids and the total amount of fluid administered to patients with ARDS is associated with improved mortality and SOFA scores.
Introduction
Acute lung disease secondary to non-cardiogenic pulmonary edema has been termed the adult respiratory distress syndrome (ARDS) since first described in 1967 by Ashbaugh et al. (1,2). ARDS was later defined at a consensus conference in Berlin (3). The Berlin definition is based on timing, chest imaging, origin of edema and oxygenation.
Despite the presence of fluid within the alveoli, it has been unclear whether a conservative strategy or liberal strategy improves outcomes. The ARDS Clinical Trials Network demonstrated that a conservative strategy based on pulmonary artery wedge pressures or central venous pressures improved lung function and shortened the duration of mechanical ventilation although there was no mortality benefit (4). However, whether fluid replacement with colloid or crystalloid in ARDS results in better outcomes remains unknown.
Recently, there have been reports of increased mortality with the use of hydroxylethyl starch (HES) in sepsis (5). Because sepsis is the most common cause of ARDS (1) this caused us to examine the use of colloids in ARDS. We found that use of colloids was associated with clinically worsening and increases mortality compared to low volumes of crystalloid in ARDS.
Materials and Methods
Subjects
This was an observational study of ARDS patients admitted to the intensive care unit (ICU) of the Norman Bethune College of Medicine, Jilin University Second Hospital, Changchun, China was conducted from November 1, 2011 to November 1, 2012.
ARDS was defined using the Berlin criteria (3).
Study Procedures
Patients were randomly divided into two groups. In one group patients were administered both crystalloid and colloid for the first 3 days of their ICU admission with ARDS. In the other group only crystalloid was used. The use of which colloid and the volume administered was left to the clinical discretion of the attending physician based on the clinical needs of the patient. Other treatment modalities such as the mode of ventilation and nutritional support were also left to the discretion of the patient although the tidal volume was kept < 7ml/kg.
Data was collected for the first 3 days of admission to the ICU. Clinical data recorded included sequential organ failure assessment (SOFA) scores, the use and amount of colloid or crystalloid, duration of ICU stay, ventilator days, need for continuous renal replacement therapy (CRRT), lactate and PaO2/FIO2. When patients received both colloid and crystalloid, volume was calculated as the sum of the volume of each. Mortality was the 90 day mortality rate.
Statistics
The data was recorded and compared using SPSS software and reported as mean + standard deviation. Comparisons between groups were performed by Student’s t-test. P values of less than 0.05 were considered significant.
Results
Patients. There were 43 patients (20 F, 23 M). The mean age was 62.7 + 18.9 years (range 20 to 85 years). The causes of ARDS was serious lung infection in 16 patients, sepsis in 9 patients, trauma in 2 patients, and pancreatitis in 2 patients. The cause was unknown in 14 patients.
Volume of fluid. The results with differing volumes of fluid administered in the first 24 hours are shown in Table 1. [Editor's note: It may be necessary to enlarge the view on your browser in order to adequately display the tables.]
Table 1. Results based on volume of fluid used in the first day.
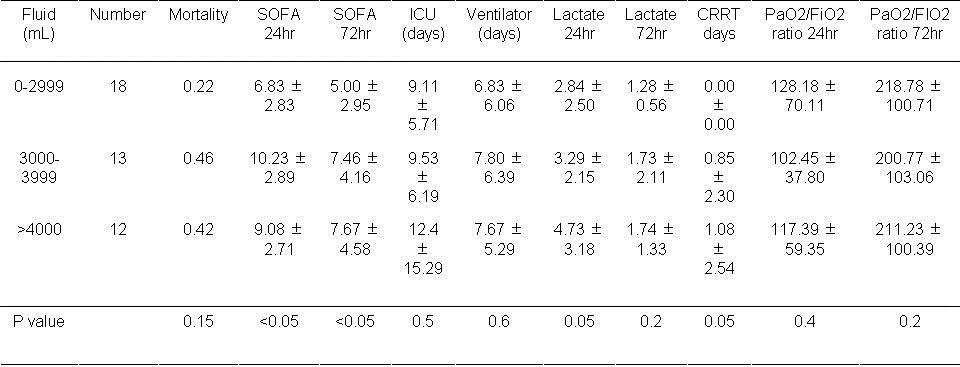
Mortality was unaffected by the volume of fluid used in the first 24 hours. However, the SOFA score at 24 and 72 hours was increased with volumes >3000 ml administered during the first 24 hours (P<0.05, both comparisons). The lactate level and the frequency of CRRT approached significance when volumes of >3000 ml were administered during the first 24 hours (P=0.05, both comparisons).
The results with volumes of greater or less than 8 liters are shown in table 2.
Table 2. Results based on volume of fluid used in the first 72 hours.
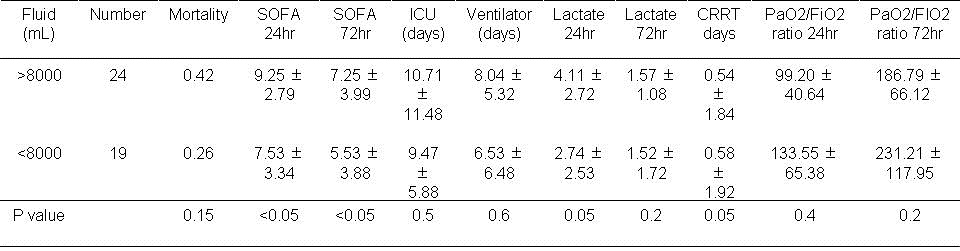
There were no significant effects of administration of greater or less than 8000 ml over 72 hours.
Type of fluid. Patients who received both crystalloid and colloid received 30 + 5% of the total volume as colloid during the first day of ICU admission. The results of administration of crystalloid compared to crystalloid and colloid during the first 24 hours are shown in Figure 3.
Table 3. Results based on type of fluid used in the first day of ICU admission.
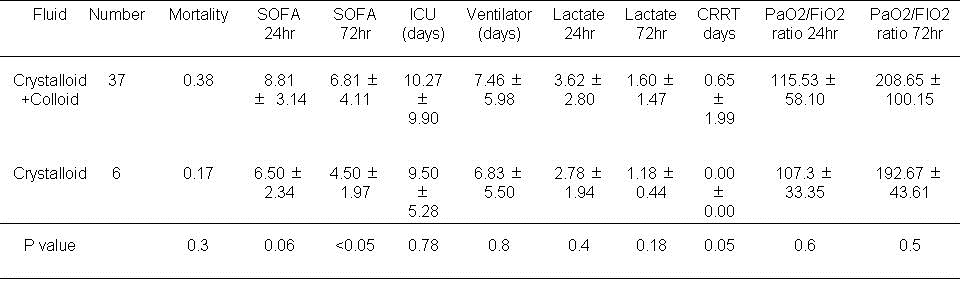
There was no difference in mortality. The use of crystalloid alone was associated with a lower SOFA score at 72 hours (P<0.05). CRRT was more often needed for those patients given both crystalloid and colloid during the first 24 hours (P=0.05).
The results when albumin was used during the first 24 hours are in Table 4.
Table 4. Results based on albumin usage during the first day.
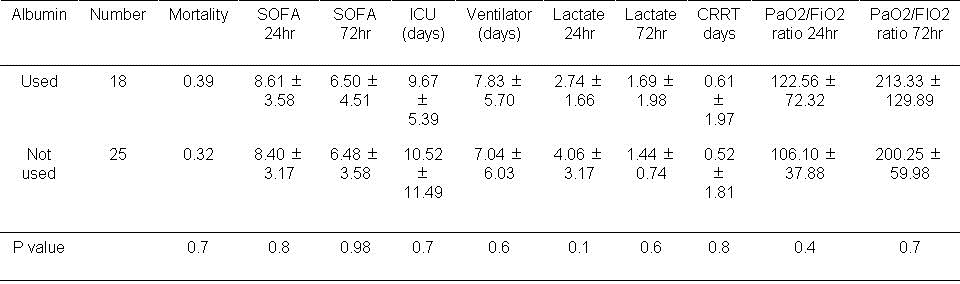
There was no significant effect on any of the measured outcomes when albumin was used in the first 24 hours.
The results with the use of plasma during the first 24 hours are shown in Table. 5.
Table 5. Results based on plasma usage during first day.
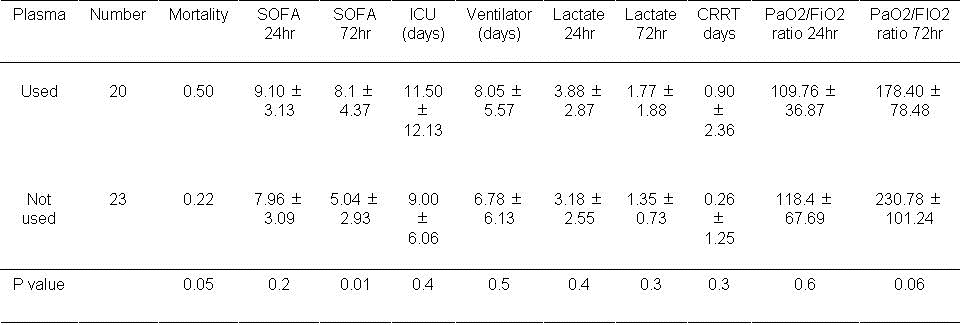
An increase in mortality approached statistical significance if plasma was used during the first 24 hours (P=0.05). The SOFA score was significantly higher at 72 hours if plasma used during the first day (P=0.01). The remaining outcomes were unchanged.
Results with hydroxylethyl starch (HES) use are shown in Table 6.
Table 6. Results based on hydroxylethyl starch (HES) usage during the first day.
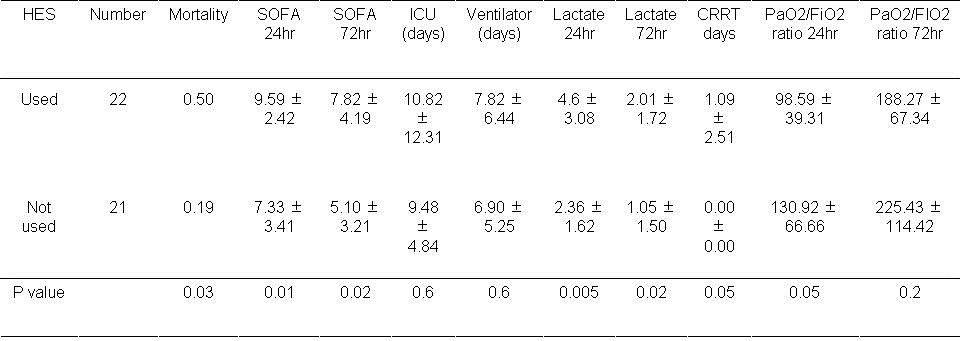
Mortality was significantly higher if HES was used during the first 24 hours (P<0.05). In addition the SOFA scores were significantly higher at 24 and 72 hours if HES was administered during the first 24 hours (P<0.05, both comparisons). The lactate level was also significantly higher at 24 hours and 72 hours (P<0.05, both comparisons). The need for CRRT and the PaO2/FiO2 ratio approached significance (P=0.05, both comparisons).
Volume of colloid. The use of colloids affected several outcomes. Therefore, the amount of colloid used was examined (Table 7).
Table 7. Results based on volume of colloid used during first day.
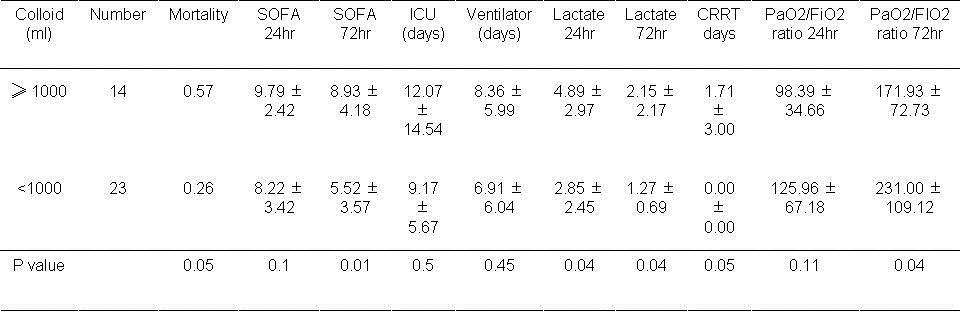
If colloid was used, mortality approached significance based on the volume of colloid used during the first 24 hours (P=0.05). Higher volumes of administered colloid (≥1000ml) were associated with a higher SOFA score at 72 hours (P=0.01). Lactate levels were significantly higher at 24 and 72 hours if colloid was used (P=0.04, both comparisons).PaO2/FiO2 was lower higher volumes of colloid usage (P=0.04).
Plasma, albumin or plasma substitutes during the first 72 hours. Some outcomes were higher with the use of colloids during the first 24 hours. Therefore, usage of plasma or albumin during the first 3 days was examined (Table 8).
Table 8. Effect of using of plasma or albumin during the first 3 days.
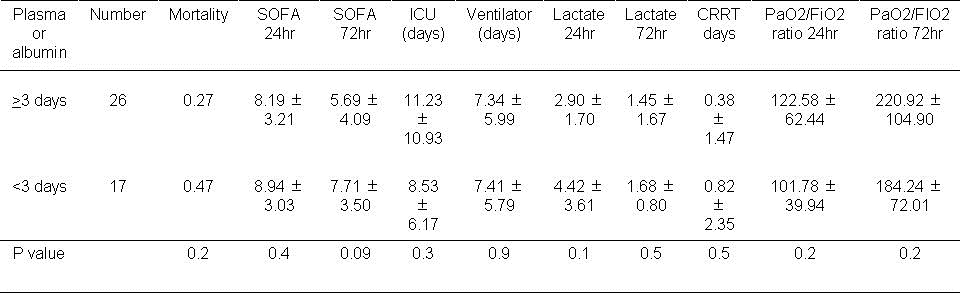
There was no significant effect on any of the outcomes with the use of plasma or albumin during the first 72 hours.
The effects of plasma substitutes during the first 72 hours are shown in Table 9.
Table 9. Results of using plasma substitutes during the first 3 days.
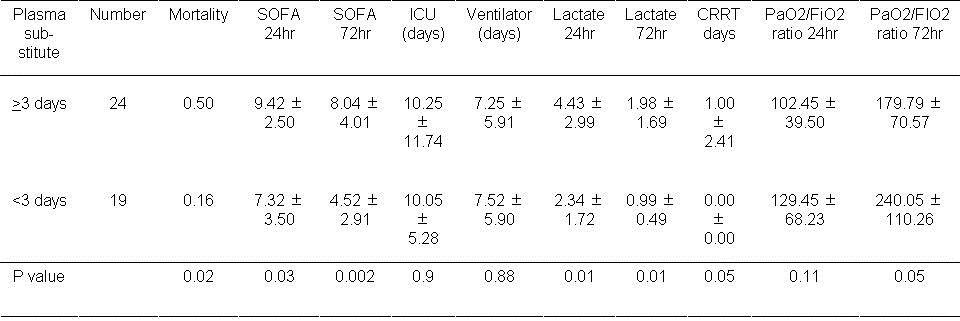
Higher mortality was associated with the use of plasma substitutes during the first 3 days (P=0.02). SOFA scores at 24 and 72 hours were also increased with plasma substitute usage (P=0.03 and P=0.002 respectively). Higher Lactate levels were also observed at 24 hours and 72 hours (P<0.01, both comparisons).
Discussion
In the hospital setting there are two types of fluid physicians administer to patients-colloid or crystalloid. Crystalloid is easily accessible and can be stored at room temperature. The main purpose of this study was to compare the two different fluids and the volume fluid used. We found that use of certain colloids, particularly higher volumes, was associated with increased mortality and poorer SOFA scores.
There was increased mortality with HES and plasma substitutes and plasma approached statistical significance. This is consistent with studies done in sepsis where HES has been associated with increased mortality (5). In contrast, there was no increase in mortality with albumin or adverse clinical outcomes, suggesting it was safe to use. The mechanism accounting for the adverse effects of colloids in ARDS and sepsis is unknown. However, the pharmokinetics of HES is known to be different from albumin and may play a role in the mortality rates (6).
We found that administration of smaller volumes of fluid was associated with improved outcomes. This confirms previous studies done in ARDS demonstrating that a conservative strategy improves outcomes. Like the ARDS Network larger, multi-center study out smaller study was unable to find a reduction in mortality with lower volumes of fluid used. From our studies it is unclear whether volume or the type of fluid is most important in determining survival. The data would seem to suggest that both are important. We also did not correct for differences in the equivalency of colloid compared to crystalloid solutions. Some authorities suggest that the volume expansive of colloid exceed crystalloid on absolute volume basis (7). However, corrections would likely accentuate the differences in mortality seen with volume.
It had been proposed that colloid infusion might be protective of the lungs by retaining fluid in the vascular space by oncotic pressure. However, recent studies have suggested show that colloids do not lower lung water (8). Furthermore, a recent meta-analysis found no evidence trials that resuscitation with colloids reduces the risk of death, compared to resuscitation with crystalloids, in patients with trauma, burns or following surgery (9). Furthermore, the use of hydroxyethyl starch might increase mortality. Our study is consistent with these studies.
Our trial has certain limitations. First, our study was single center. Second, the design did not include hemodynamic monitoring or other therapies. How these confounding variables might have affected the results is unknown. Third, only 43 patients were included in the trial. The trial was underpowered and confirmation of the results will be needed by larger trials.
This study demonstrates that the initial volume of fluid administered has effects on outcomes in patients with ARDS. The data in this manuscript support a dry or conservative strategy for management of ARDS. Furthermore, the choice of fluid also affects outcomes. The data in this paper would recommend the maintenance of relatively stable blood pressure with low volumes of crystalloid. As colloids are not associated with an improvement in survival, are less readily available, and are more expensive than crystalloids, it is hard to see how their continued use in clinical practice can be justified.
Conflict of Interest
None of the authors declared a conflict of interest.
Acknowledgements
The concept of this research was built by Prof. Dr. Yong –Jie Yin and Dr Sanjaya Karki. However, most of the credit goes to Dr Jing Xiao Zhang in order to complete this research successfully. All the other co-authors have equally contributed.
References
- Matthay AM, Zimmerman AG. Acute lung injury and the acute respiratory distress syndrome. Am J Respir Cell Mol Biol. 2005;33(4),319-327. [CrossRef] [PubMed]
- Ashbaugh DG, Bigelow DB, Petty TL, Levine BE. Acute respiratory distress in adults. Lancet. 1967;2(7511):319-23. [CrossRef] [PubMed]
- ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526-33. [CrossRef] [PubMed]
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network, Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, deBoisblanc B, Connors AF Jr, Hite RD, Harabin AL. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354(24):2564-75. [CrossRef] [PubMed]
- Perner A, Haase N, Guttormsen AB, et al. Hydroxyethyl starch 130/0.42 versus Ringer's acetate in severe sepsis. N Engl J Med. 2012;367(2):124-34. [CrossRef] [PubMed]
- Bellmann R, Feistritzer C, Wiedermann CJ. Effect of molecular weight and substitution on tissue uptake of hydroxyethyl starch: a meta-analysis of clinical studies. Clin Pharmacokinet 2012;51:225-36. [CrossRef] [PubMed]
- The SAFE Study Investigators. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med 2004;350:2247-2256. [CrossRef] [PubMed]
- van der Heijden M, Verheij J, van Nieuw Amerongen GP, Groeneveld AB. Crystalloid or colloid fluid loading and pulmonary permeability, edema, and injury in septic and nonseptic critically ill patients with hypovolemia. Crit Care Med. 2009;37(4):1275-81. [CrossRef] [PubMed]
- Perel P, Roberts I, Ker K. Colloids versus crystalloids for fluid resuscitation in critically ill patients. Cochrane Database Syst Rev. 2013 Feb 28;2:CD000567. [CrossRef] [PubMed]
Reference as: Karki S, Yin Y-J, Zhang J-X, Samani N, Pradhan D, Deuja SS, Karki R, Thakur R, Zhao N. Fluid in the management of the acute respiratory distress syndrome. Southwest J Pulm Crit Care. 2013;6(6):289-98. doi: http://dx.doi.org/10.13175/swjpcc044-13 PDF


Reader Comments