Bhupinder Natt MD
Linda Snyder MD
Janet Campion MD
University of Arizona Medical Center
Tucson, AZ
History of Present Illness
A 41 year-old man was admitted with a five-day history of cough, shortness of breath, and fever to 102° F. He was recently diagnosed with a high-grade astrocytoma of the brain and had undergone resection followed by chemotherapy with temozomide (an alkylating agent) and radiation therapy.
PMH
-
Renal transplantation (1993)
-
Glioblastoma (astrocytoma grade 4)
-
Crohn’s disease treated with budesonide and meselamine
Medications
-
Dexamethasone 2 mg PO BID
-
Keppra 500 mg PO BID
-
Tacrolimus 1.5 mg PO AM and 1mg PO PM
-
Mycophenolate 750 mg PO BID
-
Budesonide 3 mg PO daily
-
Meselamine 1600 mg PO TID
-
Sulfamethoxazole/trimethoprim DS PO on Mon/Wed/Fri
-
Temozolomide 75 mg IM with radiotherapy
Social History
Nonsmoker, no ethanol or recreational drugs, no recent travel, and no occupational exposures.
Physical Examination
T 38.6°C, P 112 beats/min, RR 32-40 breaths/min, BP 119/76 mm Hg, SpO2 100% on NRB
General: Fatigued, ill appearing and dyspneic.
Skin: No rash or lesions, well-healed craniotomy scar
HEENT: Dry oral mucosa, pupils and extra-ocular muscles normal
Respiratory: Reduced breath sounds, fine crackles throughout all lung fields, no wheezing
CVS: Hyperdynamic precordium, tachycardia without murmur, no elevation of jugular venous pressure (JVP), peripheral vascular exam normal.
Abdomen: Soft, non-distended, no hepato-splenomegaly, normal bowel sounds.
Lymph: No cervical lymphadenopathy
Extremities: No edema, normal muscle bulk and tone.
Laboratory
WBC 11 X 103/µL, Hemoglobin 9.8 g/dL, Hematocrit 30%, Platelets 264,000/ µL
Na+ 135 meq/L, K+ 4.2 meq/L, Cl− 111 meq/L, CO2 14 mmol/L, blood urea nitrogen (BUN) 46 mg/dL, creatinine 1.7 mg/dL, glucose 132 mg/dL, calcium 10.5 mg/dL, albumin 1.5 g/dL, liver function tests-within normal limits
Prothrombin time (PT) 15 sec, international normalized ratio (INR) 1.2, partial thromboplastin time (PTT) 29.9 sec
Chest X-ray
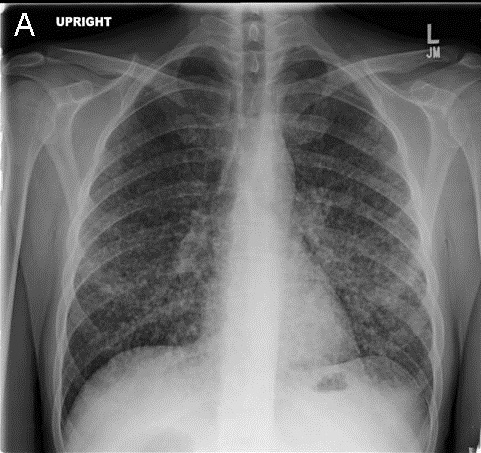

Figure 1. Admission PA (Panel A) and lateral (Panel B) chest x-ray.
What is the best description of the chest x-ray? (click on correct answer to move to next panel)
- Bibasilar consolidation
- Bilateral diffuse nodules
- Pneumomediastinum with subcutaneous emphysema
- Pulmonary edema with evidence of pulmonary hypertension
- Subdiaphragmatic free air
Reference as: Natt B, Snyder L, Campion J. January critical care case of the month: bad cough. Southwest J Pulm Crit Care. 2014;8(1):20-6. doi: http://dx.doi.org/10.13175/swjpcc161-13 PDF
 Saturday, January 4, 2014 at 8:00AM
Saturday, January 4, 2014 at 8:00AM