July 2011 Pulmonary Journal Club
 Thursday, August 4, 2011 at 9:02AM
Thursday, August 4, 2011 at 9:02AM Reference as: Mathew MJ. July, 2011 pulmonary journal club. Southwest J Pulm Crit Care 2011;3:25-6. (Click here for PDF version)
Donohue JF, Fogarty C, Lötvall J, Mahler DA, Worth H, Yorgancioglu A, Iqbal A, Swales J, Owen R, Higgins M, Kramer B; INHANCE Study Investigators. Once-Daily Bronchodilators for Chronic Obstructive Pulmonary Disease: Indacaterol Versus Tiotropium. Am J Resp Crit Care 2010;182:155-162. (Click here for full manuscript)
The once-daily, long-acting, beta-2 agonist, indacaterol was initially released in Europe in 2009. On July 1st 2011 it was approved by the FDA for the United States under the trade name Arcapta Neoinhaler. There have been studies demonstrating Indacaterol as an efficacious alternative to twice daily bronchodilators such as formoterol (1). We reviewed the study published by Donahue et al. and examined how Indacaterol compared to the only other once daily long acting bronchodilator tiotropium.
This was a large, two stage, randomized, controlled study performed between April, 2007 – August, 2008. The inclusion criteria were an age > 40, smoking history of > 20 pack/yrs and GOLD criteria COPD of moderate – severe COPD. The study was performed in 2 stages. A total of 2059 patients were included in the 1st stage which was performed to test dose efficacy. In this stage patients were randomized to receive either once daily indacaterol at doses of 75 ug, 150 ug, 300 ug or 600 ug, formoterol, tiotropium or placebo. At the end of this stage, two doses of indacaterol were selected for the second stage based on efficacy and safety. In the second stage, 1683 patients were randomized to once daily indacaterol 150 ug, 300 ug, tiotropium or placebo.
Primary endpoints were FEV1 response against placebo at 24 hours, and again at 12 weeks. The secondary endpoint was to compare FEV1 at 12 weeks against
tiotropium. A total of 1291 patients completed the study. The results showed there was a statistically significant increase in FEV1 at 24hrs and 12 weeks when compared to placebo. The study also demonstrated non-inferiority to tiotropium at 12 weeks with regards to improvement in FEV1 (see table below).
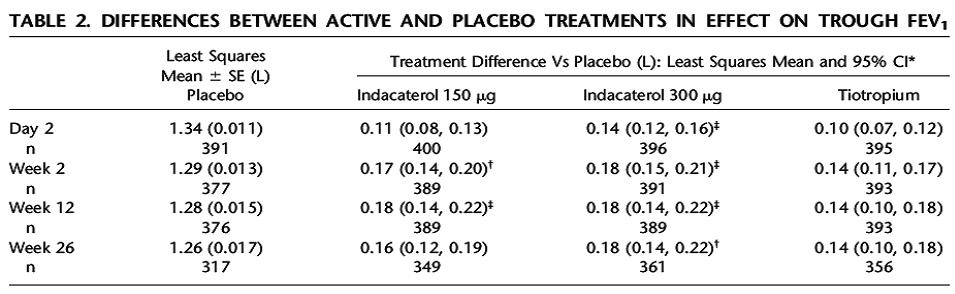
The study was not designed to measure clinical outcomes but noted were a decrease in the use of daily albuterol, a decrease in daytime symptoms and nocturnal awakenings in the indacaterol arm vs. placebo. The medication was well tolerated with main side effects being reported as cough, tachycardia and dry mouth.
The study was well done and it accomplished its primary endpoint. The main limitations were the lack of blinding in the tiotropium arm and the relative short study period of 26 weeks. It would have also been useful to extend the study period to 1 year to obtain clinical outcome data.
The availability of a once-daily, long-acting beta-2 agonist is now a promising alternative in the treatment of COPD. Although more studies are needed to demonstrate an improvement in clinical outcomes (such as a reduction in COPD
exacerbations) the once a day dosing offers at the very least, a compliance advantage. The cost of the medication (which is currently unknown) and formulary restrictions will limit initial availability.
Manoj Mathew, MD MCCM, FCCP
References
1. Dahl R, Chung KF, Buhl R, et al. Efficacy of a new once-daily long-acting inhaled beta2-agonist indacaterol versus twice-daily formoterol in COPD. Thorax 2010;65:473–9.
 COPD,
COPD,  FEV1,
FEV1,  indacaterol,
indacaterol,  tiotropium
tiotropium 