Layth Al-Jashaami, MD
Yousef Usta, MD
Negin N. Blattman, MD
Rakesh Nanda, MD
Phoenix VA Health Care System
650 E Indian School Road
Phoenix, Arizona, 85012 USA
Critical Care Case of the Month CME Information
Members of the Arizona, New Mexico, Colorado and California Thoracic Societies and the Mayo Clinic are able to receive 0.25 AMA PRA Category 1 Credits™ for each case they complete. Completion of an evaluation form is required to receive credit and a link is provided on the last panel of the activity.
0.25 AMA PRA Category 1 Credit(s)™
Estimated time to complete this activity: 0.25 hours
Lead Author(s): Layth Al-Jashaami, MD. All Faculty, CME Planning Committee Members, and the CME Office Reviewers have disclosed that they do not have any relevant financial relationships with commercial interests that would constitute a conflict of interest concerning this CME activity.
Learning Objectives:
As a result of this activity I will be better able to:
- Correctly interpret and identify clinical practices supported by the highest quality available evidence.
- Will be better able to establsh the optimal evaluation leading to a correct diagnosis for patients with pulmonary, critical care and sleep disorders.
- Will improve the translation of the most current clinical information into the delivery of high quality care for patients.
- Will integrate new treatment options in discussing available treatment alternatives for patients with pulmonary, critical care and sleep related disorders.
Learning Format: Case-based, interactive online course, including mandatory assessment questions (number of questions varies by case). Please also read the Technical Requirements.
CME Sponsor: University of Arizona College of Medicine
Current Approval Period: January 1, 2015-December 31, 2016
Financial Support Received: None
History of Present Illness
A 50-year-old African American woman presented with weakness, altered mental status and constipation of 12 days duration. She was complaining of abdominal distension with diffuse pain and bloating. She denied melena, hematochezia or hematemesis. She had a history weight loss, anorexia and fatigue which had evolved over the past few months leading to recent severe weakness and inability to get out of bed.
Past Medical History, Social History and Family History
Her past medical history included HIV infection with AIDS and noncompliance with her antiretroviral medications. Her most recent CD4 count was <20 cells/uL and viral load of 554,483 copies/mL.
Physical Examination
Vital signs: Blood pressure, 120/80 mmHg, heart rate, 105/min, temperature, 98.6° and respiratory rate, 20/min.
General: Physical examination showed a lethargic female who was poorly responsive to questioning.
Abdomen: Distended, tympanic abdomen with hypoactive bowel sounds and diffuse tenderness.
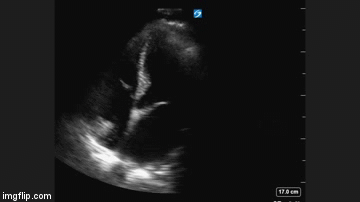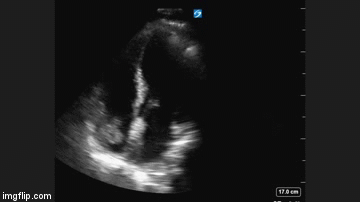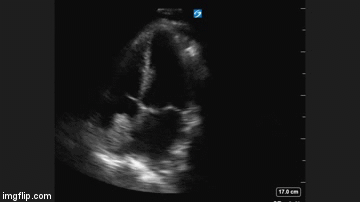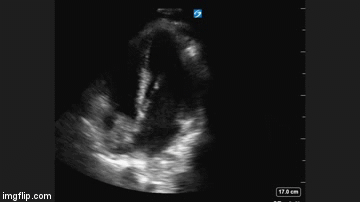
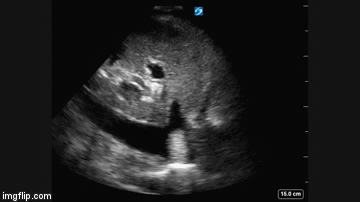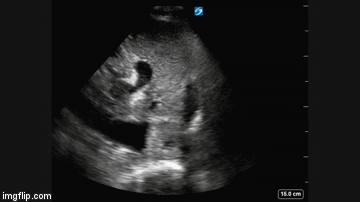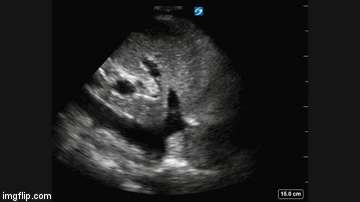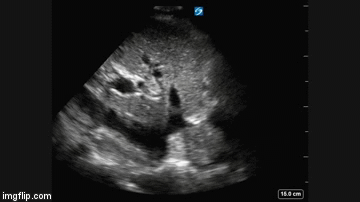
Radiography
Plain x-ray examination of the abdomen on admission is shown in Figure 1.
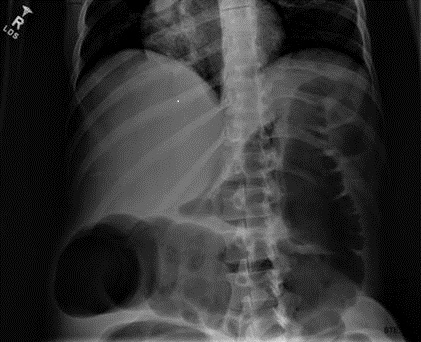
Figure 1. Admission x-ray of the abdomen.
Which of the following are possible causes of the patient's complaints, physical findings and abdominal x-ray findings? (Click on the correct answer to proceed to the second of six panels)
- Electrolyte disturbances
- Use of anticholinergic drugs
- Use of narcotics
- 1 and 3
- All of the above
Cite as: Al-Jashaami L, Usta Y, Blattman NN, Nanda R. May 2016 critical care case of the month. Southwest J Pulm Crit Care. 2016 May;12(5):171-9. doi: http://dx.doi.org/10.13175/swjpcc038-16 PDF
 Monday, July 4, 2016 at 8:00AM
Monday, July 4, 2016 at 8:00AM