Bhargavi Gali, M.D.1
Grace M. Arteaga, M.D.2
Glen Au, R.N., C.C.R.N.3
Vitaly Herasevich, M.D., Ph.D.1
1Division of Anesthesia-Critical Care Medicine, Department of Anesthesiology and Perioperative Medicine
2Division of Pediatric Critical Care Medicine, Department of Pediatric and Adolescent Medicine
3Department of Nursing
Mayo Clinic
Rochester, Minnesota USA
Abstract
Background: Advanced life support interventions have been modified for patients who have recently undergone sternotomy for cardiac surgery and have new suture lines. We aimed to determine whether the use of in-situ simulation increased adherence to the cardiac surgery unit-advanced life support algorithm (CSU-ALS) for patients with cardiac arrest after cardiac surgery (CAACS).
Methods: This was a retrospective chart review of cardiac arrest management of patients who sustained CAACS before and after implementation of in-situ simulation scenarios utilizing CSU-ACLS in place of traditional advanced cardiac life support. We utilized classroom education of CSU-ACLS followed by in-situ high-fidelity simulated scenarios of patients with CAACS.. Interprofessional learners (n = 210) participated in 18 in-situ simulations of CAACS. Two groups of patients with CAACS were retrospectively compared before and after in situ training (preimplementation, n=22 vs postimplementation, n=38). Outcomes included adherence to CSU-ALS for resuscitation, delay in initiation of chest compressions, use of defibrillation and pacing before external cardiac massage, and time to initial medication.
Results: Chest compressions were used less often in the postimplementation vs the preimplementation period (11/22 [29%] vs 13/38 [59%], P = 0.02). Time to initial medication administration, use of defibrillation and pacing, return to the operating room, and survival were similar between periods.
Conclusion: In this pilot, adherence to a key component of the CSU-ALS algorithm—delaying initiation of chest compressions—improved after classroom combined with in-situ simulation education.
Abbreviations
- ACLS, advanced cardiac life support
- CAACS, cardiac arrest after cardiac surgery
- CPR, cardiopulmonary resuscitation
- CSICU, cardiac surgical intensive care unit
- CSU-ALS, cardiac surgical unit–advanced life support EACTS, European Association for Cardio-Thoracic Surgery
- IQR, interquartile range
- STS, Society of Thoracic Surgeons
Introduction
Immediate and appropriate resuscitation of patients with cardiac arrest has been called “the formula for survival” (1). Patient-specific and cause-specific resuscitation algorithms have been developed to optimize management and outcome measures (2). Advanced cardiac life support (ACLS) interventions are modified for special causes, environments, and patient populations. Patients who have recently undergone sternotomy for cardiac surgery and have new suture lines is one of these groups.
Because of their unique circumstances and physiologic conditions, patients who have recently undergone cardiac surgery benefit from modified cardiac-arrest management protocols. A recent consensus guideline by The Society of Thoracic Surgeons (STS) recommends use of a postcardiac surgery–specific resuscitation protocol prepared by the European Association for Cardio-Thoracic Surgery (EACTS), hereafter called the STS/EACTS protocol (3). In contrast to ACLS guidelines(4), the STS/EACTS protocol is based on recent sternotomy and increased risks of cardiac tamponade and cardiac ventricular rupture. The STS/EACTS protocol recommends sequential attempts at defibrillation before administration of chest compressions, administration of low-dose epinephrine, use of pacing to manage severe bradycardia or asystole, and immediate consideration of resternotomy (Table 1).
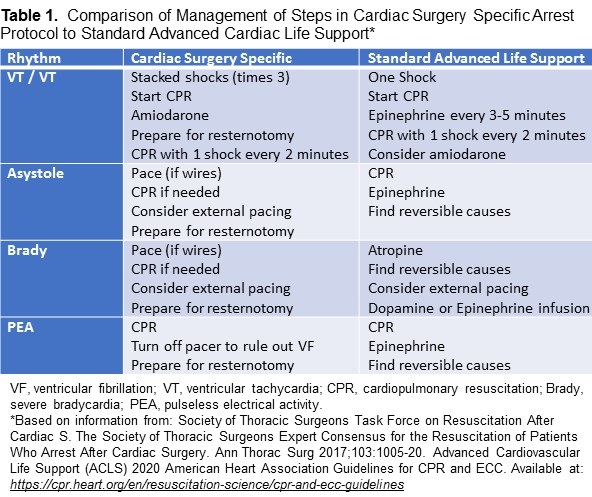
Because poststernotomy patients have new suture lines, they are at risk for comorbid conditions (e.g., cardiac tamponade, ventricular rupture) if external chest compressions are used (4). The cardiac surgical unit–advanced life support (CSU-ALS) protocol emphasizes use of defibrillation and delayed use of chest compressions (Table 1). In-situ simulation-based education has been shown to be an effective method for training in high-risk, low-frequency resuscitation situations (5). During in-situ simulation-based education, health care providers receive training in their clinical work environment.
A systematic review and meta-analysis of 182 studies reported that simulation-based training was highly effective in improving knowledge and process skill (6).
The STS/EACTS protocol was introduced to the CSICU in April 2014. The CSICU team members, who all had background training in ACLS, received classroom-based education on the application of the cardiac surgery unit–advanced life support (CSU-ALS) algorithm. In-situ simulation-based training with resuscitation scenarios offered the team members the experimental application of the STS/EACTS resuscitation protocol-CSU-ALS protocol. We hypothesized that adherence to the CSU-ALS protocol for the treatment of patients with CAACS would improve after a pilot implementing in-situ simulations with our CSICU team members.
Methods
After obtaining approval from the Mayo Clinic Institutional Review Board, we performed a single-center, retrospective review of the electronic health records of patients with CAACS. Only the records of patients that had consented to have their data utilized for research were included. The CONSORT 2010 Checklist was utilized in preparation of this manuscript. We identified patients who were treated before (October 2013 through March 2014; preimplementation period) or after simulation training (October 2015 through March 2016; postimplementation period). technicians; in total, 210 participants, took part in 18 simulations. All participants, except the pharmacists, respiratory therapists, and phlebotomists, had participated in CSU-ALS classroom education. No repeat participants were included in these sessions. A combined 35% of our CSICU staff participated.
Included patients were those admitted to the CSICU after sternotomy for cardiac surgery, specifically patients who had undergone sternotomy and a cardiac surgical procedure (including those who underwent initiation of central extracorporeal membrane oxygenation). Patients from the above group who had cardiac arrest within the first 14 days after sternotomy for cardiac surgery were included. We excluded inpatients who were in the CSICU 14 days after their original sternotomy at time of cardiac arrest.
The educational in-situ simulations portrayed adult patients with cardiac arrest immediately after cardiac surgery. The details of the simulation have been previously published (7). Briefly, the learning objectives were established according to the CSU-ALS protocol. Before the simulation, a facilitator familiar with the CSU-ALS protocol reviewed it with the participants and discussed the differences compared to ACLS. Cardiopulmonary resuscitation (CPR) was defined as basic life support with use of the ACLS algorithm, airway management, greater epinephrine doses, and chest compressions initiated immediately after rhythm check; ACLS included all algorithms used in resuscitation, as recommended by the American Heart Association (4). In contrast to ACLS, CSU-ALS emphasizes the need to initially defibrillate rather than to perform chest compressions. A patient room inside the CSICU was used as the scenario set-up. A high-fidelity mannequin was endotracheally intubated and mechanically ventilated. The simulation timeline involved 10 to 15 minutes for the case development and followed by a reflective debriefing period of 10 to 15 minutes.
The participating interprofessional team included critical care nurses, critical care fellows, cardiac surgical fellows, critical care physicians, pharmacists, nurse practitioners, respiratory therapists, and phlebotomy participants were included in these sessions. A combined 35% of our CSICU staff participated.
We collected data on patient demographic characteristics, surgical procedures and dates, specific cardiac arrest characteristics (initial cardiac rhythm and presumed cause), and resuscitation characteristics (return to the operating room for resternotomy [yes or no], intubation [yes or no], and survival of event [yes or no]).
The primary outcome measure in our scenarios was the use of defibrillation with successive “stacked” shocks prior to the standard ACLS, which recommends immediate initiation of chest compressions (7). Secondary outcome measures included time to initiation of chest compressions, time to use of ventricular defibrillation and pacing, and time to initial medication administration.
Statistical Analysis
Results are reported with descriptive statistics. All continuous variables are summarized as median (interquartile range [IQR]) or mean (SD) as appropriate, and we used the Wilcoxon rank sum test to compare the means and medians of continuous variables. Categorical data are summarized as number (percentage), and we used the Fisher’s exact test to compare categorical variables. Two-tailed hypothesis testing was used, and P < 0.05 was considered significant. Analysis was performed with JMP Pro 14.1.0 (SAS Institute Inc; Cary, North Carolina) and Microsoft Excel 2010 version 14 (Microsoft Corp; Redmond, Washington).
Results
Sixty patients met the inclusion criteria. We identified 22 patients in the preimplementation period (10 women, 45%) and 38 patients in the postimplementation period (12 women, 32%). In the preimplementation group, 6/22 patients (27%) received extracorporeal membrane oxygenation, compared with 8/38 patients (21%) in the postimplementation group. Initial presentation and etiology of the arrests in the pre- and postimplementation period are presented in Table 2.
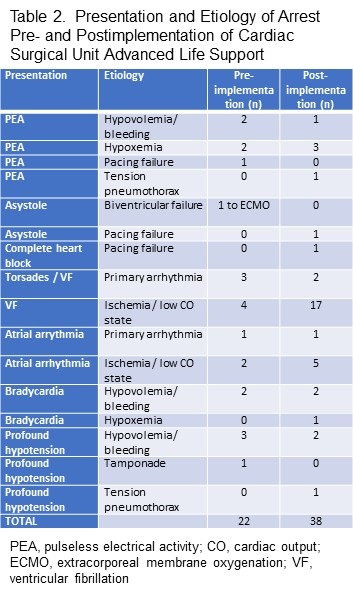
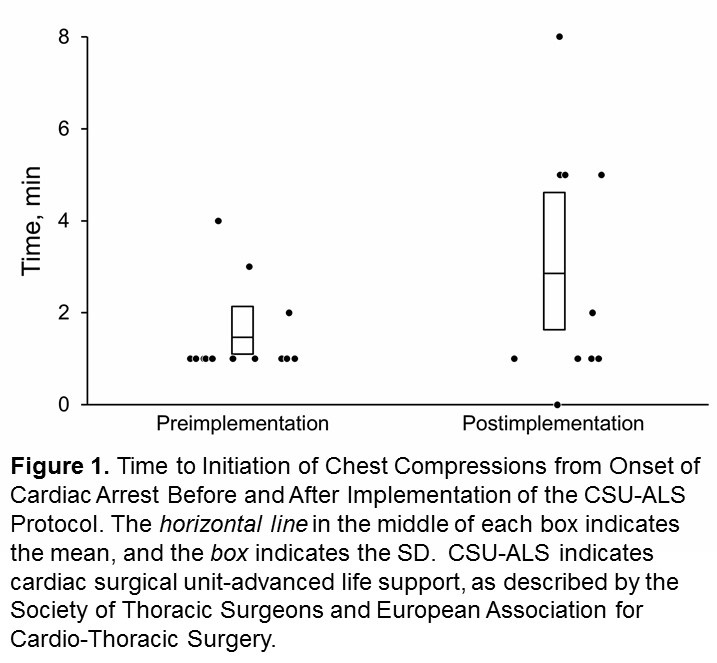
The use of chest compressions was 59% (preimplementation: 13/22 patients) vs 29% (the postimplementation phase 12/38 patients) (P = 0.02) and standard CPR (22/22 patients [100%] vs 27/38 patients [71%], P < 0.001) respectively (Table 2). Median (IQR) time from onset of cardiac arrest to initiation of chest compressions was 1 minute (1-1.5 minutes) in the preimplementation period and 1.5 minutes (1-5 minutes) in the postimplementation period; these findings were statistically similar (P = 0.11) (Figure 1).
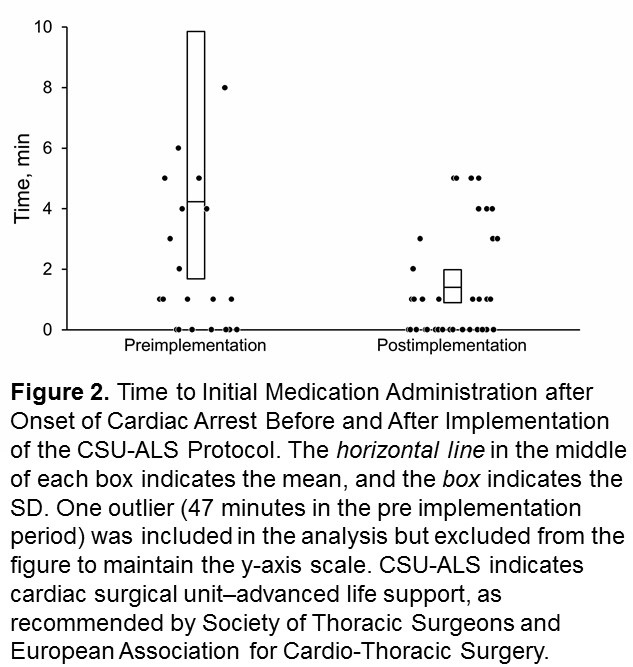
Median time to initial medication administration was similar between periods (P = 0.11). However, in the preimplementation period, one patient was administered medication 47 minutes after cardiac arrest. This result was an outlier (Figure 2).
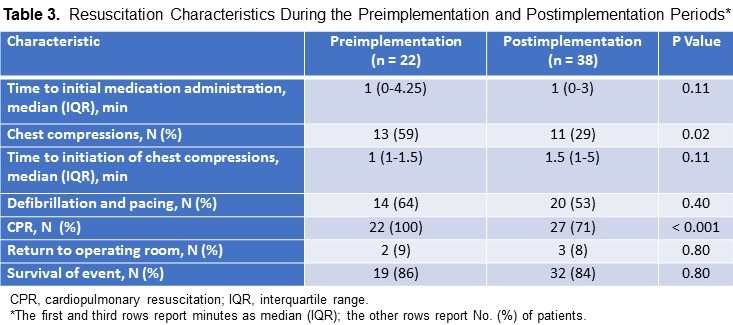
Similar percentages of patients received defibrillation to manage ventricular fibrillation or tachycardia (14/22 patients [64%] in the preimplementation period vs 20/38 patients [53%] in the postimplementation period, P = 0.40), returned to the operating room for resternotomy (2/22 patients [9%] vs 3/38 patients [8%], P = 0.80), and survived the event (19/22 patients [86%] vs 32/33 patients [84%], P = 0.80) (Table 3).
Discussion
The findings in this pilot study revealed an increase in adherence to CUS-ALS principles in CAACS when online courses are followed by in-situ simulation-based education. Our preliminary data show a decrease in the use of standard CPR and chest compressions to manage CAACS. These results suggest that in situ simulation–based training may potentially increase adherence to alternative resuscitation protocols for special patient populations and circumstances.
Mundell et al. (6) described how team training, including practice of interactions during resuscitation with provision of feedback, positively affected trainee satisfaction, knowledge, time to action, and process skill outcomes. In addition, a recent systematic review and meta-analysis of observational studies reported a positive association between participation in ACLS courses and patient outcomes, including return of spontaneous circulation (8).
The current study provides preliminary evidence that in situ simulation-based training improves clinical performance. Participation in simulation-based training allowed our CSICU team members to apply classroom-based knowledge in an experiential-learning environment, thereby improving their clinical performance of CSU-ALS protocol when they managed high-risk events.
We were able to educate our team members about a key component of the CSU-ALS protocol-namely, delay initiation of chest compressions and standard CPR. Our study did not find significant differences between groups for time to medication administration, use of defibrillation, return to the operating room, or survival. Because this study was retrospective, we were unable to determine whether our CSICU team members who participated in simulation-based training subsequently resuscitated patients after the CSU-ALS protocol was implemented at our institution. This could have affected our ability to assess the effects of in situ simulation–based training on clinical management.
Limitations
Our study has limitations is its retrospective design and involvement of 35% of staff with the in-situ simulations. Documentation of cardiac arrest has improved at our institution, but one patient in the preimplementation period had a long-documented time from cardiac arrest to initial medication administration (47 minutes); this result was an outlier and was most likely a charting error.
Another limitation was our inability to exactly determine which CSICU team members who treated patients in the postimplementation period had participated in in situ simulation-based training. based on de-identified data collection, one-third of our CSICU staff participated in this educational experience.
Due to our limited number of arrests, alterations in outcomes based on in situ simulation would not likely be noted. In situ simulation–based training improves cardiac arrest management and provides health care personnel a safe environment to practice interventions, which subsequently improves patient safety.[6, 12-14] Further prospective studies of the use of in situ simulation–based training may help determine the true effectiveness of this tool in educational and clinical practices that use specific resuscitation algorithms and highlight the relationship to patient outcomes and patient safety.
Conclusions
Analysis of the effects of in situ simulation-based training in the clinical setting showed a significant beneficial decrease in the use of chest compressions for the management of CAACS in patients who recently had undergone sternotomy. Increased adherence to the CSU-ALS protocol could improve the outcome measures of patients with CAACS and decrease the deleterious effects of chest compressions after recent sternotomy with the expectation of decreased complications and ultimately, improved clinical outcomes. As this was a small pilot study, further investigation with use of in-situ simulation in special circumstances would help determine its utility as an educational tool for high risk low frequency events.
References
- Søreide E, Morrison L, Hillman K, Monsieurs K, Sunde K, Zideman D, Eisenberg M, Sterz F, Nadkarni VM, Soar J, Nolan JP; Utstein Formula for Survival Collaborators. The formula for survival in resuscitation. Resuscitation. 2013 Nov;84(11):1487-93. [CrossRef] [PubMed]
- Truhlář A, Deakin CD, Soar J, et al. European Resuscitation Council Guidelines for Resuscitation 2015: Section 4. Cardiac arrest in special circumstances. Resuscitation. 2015 Oct;95:148-201. [CrossRef] [PubMed]
- Advanced Cardiovascular Life Support (ACLS) American Heart Association 2020 Guidelines for CPR and ECC Available at: https://cpr.heart.org/en/resuscitation-science/cpr-and-ecc-guidelines , Accessed July1, 2021.
- Society of Thoracic Surgeons Task Force on Resuscitation After Cardiac Surgery. The Society of Thoracic Surgeons Expert Consensus for the Resuscitation of Patients Who Arrest After Cardiac Surgery. Ann Thorac Surg. 2017 Mar;103(3):1005-1020. [CrossRef] [PubMed]
- Greif R, Lockey AS, Conaghan P, Lippert A, De Vries W, Monsieurs KG. European Resuscitation Council Guidelines for Resuscitation 2015: Section 10. Education and implementation of resuscitation. Resuscitation. 2015;95:288-301. [CrossRef] [PubMed]
- Mundell WC, Kennedy CC, Szostek JH, Cook DA. Simulation technology for resuscitation training: a systematic review and meta-analysis. Resuscitation. 2013 Sep;84(9):1174-83. [CrossRef] [PubMed]
- Gali B, Au G, Rosenbush KA. Simulation Incorporating Cardiac Surgery Life Support Algorithm Into Cardiac Intensive Care Unit Practice. Simul Healthc. 2016 Dec;11(6):419-424. [CrossRef] [PubMed]
- Lockey A, Lin Y, Cheng A. Impact of adult advanced cardiac life support course participation on patient outcomes-A systematic review and meta-analysis. Resuscitation. 2018 Aug;129:48-54. [CrossRef] [PubMed]
- Fernández Lozano I, Urkía C, Lopez Mesa JB, Escudier JM, Manrique I, de Lucas García N, Pino Vázquez A, Sionis A, Loma Osorio P, Núñez M, López de Sá E. European Resuscitation Council Guidelines for Resuscitation 2015: Key Points. Rev Esp Cardiol (Engl Ed). 2016 Jun;69(6):588-94. [CrossRef] [PubMed]
- Dunning J, Fabbri A, Kolh PH, Levine A, Lockowandt U, Mackay J, Pavie AJ, Strang T, Versteegh MI, Nashef SA; EACTS Clinical Guidelines Committee. Guideline for resuscitation in cardiac arrest after cardiac surgery. Eur J Cardiothorac Surg. 2009 Jul;36(1):3-28. [CrossRef] [PubMed]
- Dunning J, Nandi J, Ariffin S, Jerstice J, Danitsch D, Levine A. The Cardiac Surgery Advanced Life Support Course (CALS): delivering significant improvements in emergency cardiothoracic care. Ann Thorac Surg. 2006 May;81(5):1767-72. [CrossRef] [PubMed]
- Haffner L, Mahling M, Muench A, et al. Improved recognition of ineffective chest compressions after a brief Crew Resource Management (CRM) training: a prospective, randomised simulation study. BMC Emerg Med. 2017 Mar 3;17(1):7. [CrossRef] [PubMed]
- Edwards FH, Ferraris VA, Kurlansky PA, et al. Failure to Rescue Rates After Coronary Artery Bypass Grafting: An Analysis From The Society of Thoracic Surgeons Adult Cardiac Surgery Database. Ann Thorac Surg. 2016 Aug;102(2):458-64. [CrossRef] [PubMed]
- Mahramus TL, Penoyer DA, Waterval EM, Sole ML, Bowe EM. Two Hours of Teamwork Training Improves Teamwork in Simulated Cardiopulmonary Arrest Events. Clin Nurse Spec. 2016 Sep-Oct;30(5):284-91. [CrossRef] [PubMed]
Acknowledgements
We would like to acknowledge Robin Williams for her work on editing and formatting the manuscript.
Cite as: Gali B, Arteaga GM, Au B, Herasevich V. Impact of In Situ Education on Management of Cardiac Arrest after Cardiac Surgery. Southwest J Pulm Crit Care. 2021;23(2):54-61. doi: https://doi.org/10.13175/swjpcc028-21 PDF
 Saturday, January 1, 2022 at 8:00AM
Saturday, January 1, 2022 at 8:00AM 










