August 2016 Pulmonary Case of the Month
 Monday, August 1, 2016 at 8:00AM
Monday, August 1, 2016 at 8:00AM Anjuli M. Brighton, MB, BCh, BAO
Kathryn E. Williams, MB, BCh, BAO
Lewis J. Wesselius, MD
Pulmonary Department
Mayo Clinic Arizona
Scottsdale, AZ USA
Pulmonary Case of the Month CME Information
Members of the Arizona, New Mexico, Colorado and California Thoracic Societies and the Mayo Clinic are able to receive 0.25 AMA PRA Category 1 Credits™ for each case they complete. Completion of an evaluation form is required to receive credit and a link is provided on the last panel of the activity.
0.25 AMA PRA Category 1 Credit(s)™
Estimated time to complete this activity: 0.25 hours
Lead Author(s): Anjuli M. Brighton, MB. All Faculty, CME Planning Committee Members, and the CME Office Reviewers have disclosed that they do not have any relevant financial relationships with commercial interests that would constitute a conflict of interest concerning this CME activity.
Learning Objectives:
As a result of this activity I will be better able to:
- Correctly interpret and identify clinical practices supported by the highest quality available evidence.
- Will be better able to establsh the optimal evaluation leading to a correct diagnosis for patients with pulmonary, critical care and sleep disorders.
- Will improve the translation of the most current clinical information into the delivery of high quality care for patients.
- Will integrate new treatment options in discussing available treatment alternatives for patients with pulmonary, critical care and sleep related disorders.
Learning Format: Case-based, interactive online course, including mandatory assessment questions (number of questions varies by case). Please also read the Technical Requirements.
CME Sponsor: University of Arizona College of Medicine at Banner University Medical Center Tucson
Current Approval Period: January 1, 2015-December 31, 2016
Financial Support Received: None
History of Present Illness
The patient is 54-year-old man with type 1 diabetes mellitus admitted for diabetic ketoacidosis (DKA). He complained of somnolence, nausea and vomiting and right foot pain. He had been admitted 2 weeks earlier for right foot gangrene. He had been receiving daptomycin for his right foot gangrene.
PMH, SH and FH
He had a previous history of osteomyelitis, perianal abscess, maxillary abscess, Candida esophagitis, transient ischemic attack, and peripheral vascular disease. He had previous amputations along with thrombectomy/ embolectomy/bypass. He was a former Marine and construction worker with ongoing cigarette use. Family history was noncontributory.
Physical Examination
- Febrile to 38.2ºC
- Crackles bilaterally
- Transmetatarsal stump with dry gangrene
Radiography
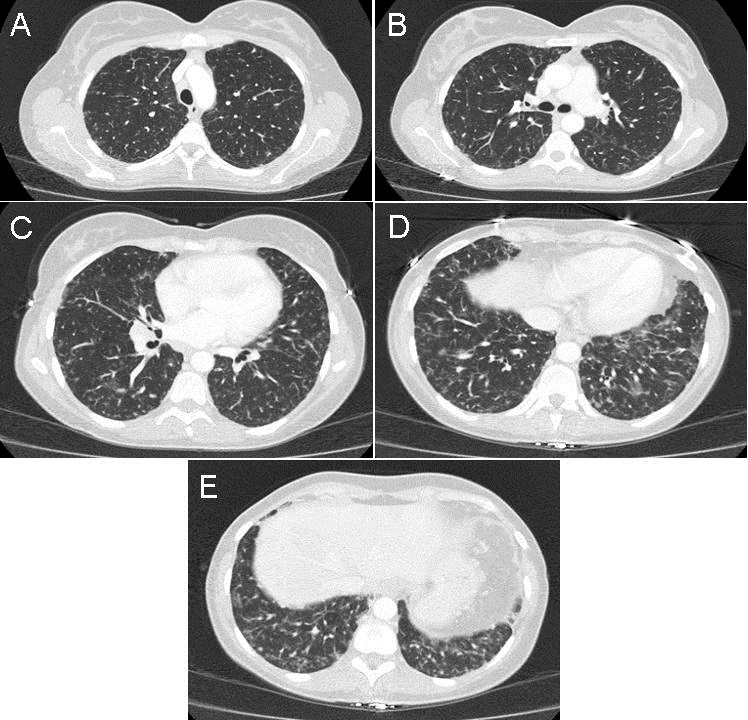
An admission chest x-ray was performed (Figure 1).

Figure 1. Admission portable AP of chest.
Which of the following are appropriate at this time? (Click on the correct answer to proceed to the second of four panels)
- Blood and wound cultures
- Empiric antibiotics including coverage for Staphylococcus aureus
- Intravenous insulin and fluids
- Serially monitor renal function and electrolytes
- All of the above
Cite as: Brighton AM, Williams KE, Wesselius LJ. August 2016 pulmonary case of the month. Southwest J Pulm Crit Care. 2016;13(2):40-5. doi: http://dx.doi.org/10.13175/swjpcc070-16 PDF