Diagnostic Challenges of Acute Eosinophilic Pneumonia Post Naltrexone Injection Presenting During The COVID-19 Pandemic
 Monday, February 14, 2022 at 8:00AM
Monday, February 14, 2022 at 8:00AM Michelle Breuer
Abdulmonam Ali, MD
SSM Health
Mount Vernon, IL USA
Introduction
Acute eosinophilic pneumonia (AEP) is a rare respiratory illness that may present with nonspecific symptoms ranging in severity from cough and dyspnea to potentially fatal acute respiratory distress syndrome. Although the exact etiology of AEP is unknown, it is thought to be a hypersensitivity reaction that can be idiopathic or caused by various infections, inhalation exposures, and medications (1). Here we present a rare case of AEP secondary to injectable naltrexone.
Case Presentation
A 45-year-old Caucasian male with a history of alcohol use disorder presented to the emergency room with a 3-day history of progressively worsening dyspnea and dry cough. The patient was a lifelong non-smoker with an unremarkable past medical history aside from alcohol abuse and obesity (BMI 41.64 kg/m²). He denied fever or chills, orthopnea, chest pain, or symptoms suggestive of paroxysmal nocturnal dyspnea. He also denied any recent sick contacts, including exposure to COVID-19. Relevant history includes alcohol cessation 1 month before presentation. After 2 weeks of cessation, he received his first injection of naltrexone (Vivitrol®) as part of alcohol relapse prevention. Physical exam was notable for an initial SpO2 of 69% on room air, sinus tachycardia at a rate of 121 bpm, and obesity. Chest examination exhibited decreased air entry with bilateral fine crackles on auscultation. No skin rashes or peripheral edema were appreciated, and the remaining physical exam was within normal limits. The patient was started on supplemental oxygen (6 liters/minute nasal cannula to maintain SpO2 above 90%).
Workup was performed and chest x-ray showed diffuse bilateral pulmonary infiltrates (Figure 1), hence, the patient was started on empiric antibiotic and steroid therapy.

Figure 1. Chest X-ray showing bilateral ground-glass opacities.
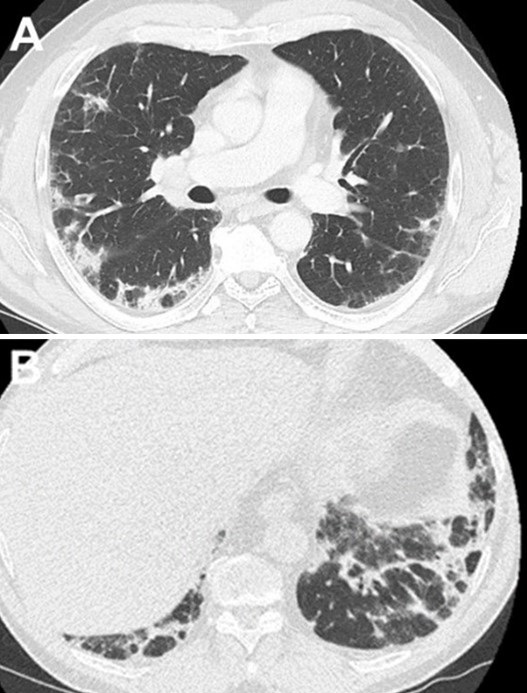
SARS-CoV-2 PCR testing was performed twice due to high clinical suspicion of COVID-19 infection (the patient was seen during the Coronavirus pandemic). Both SARS-CoV-2 tests were negative as well as the rest of the respiratory viral panel. CBC was significant for leukocytosis with an absolute peripheral eosinophil count of 0.49 x 109 cells/L. Bloodwork also revealed mildly elevated troponin, d-dimer, and LDH. However, electrocardiogram showed no significant ST changes and Computerized Tomography (CT) angiography chest showed no evidence of pulmonary embolism but confirmed the chest x-ray findings of diffuse bilateral ground-glass opacities with anterolateral subpleural parenchymal sparing (Figure 2).

Figure 2. CTA chest (axial view, lung window) showing diffuse ground-glass opacities.
An echocardiogram showed an ejection fraction of 60% and normal left ventricular diastolic function. Moderate right ventricular (RV) dilation with reduced systolic function was reported and the peak RV pressure was estimated at 39 mmHg. Extensive blood testing for connective tissue disease was negative for ANCA, CCP, ANA, and cryoglobulins. Immunoglobulin E (IgE) level was within normal limits at 14KU/L (reference range < 214 KU/L). Infectious disease serology was negative for mycoplasma, strongyloides, coccidioides, and aspergillus. HIV and hepatitis screening were also negative. Bronchoscopy with bronchoalveolar lavage (BAL) was performed and was significant for 27% eosinophils, 42% lymphocytes, 25% monocytes, 6% neutrophils (Figure 3).

Figure 3. Bronchoalveolar lavage (BAL) showing increased numbers of eosinophils.
BAL culture remained negative including mycobacterial and fungal cultures. BAL testing for Pneumocystis Jirovecii was negative as well. BAL cytology showed benign bronchial epithelial cells and inflammatory cells. No parasites were seen in BAL and fungal staining was negative.
The constellation of the above clinical, radiological, and laboratory findings was highly suggestive of acute eosinophilic pneumonia diagnosis. The patient’s methylprednisolone dose was increased to 125mg every 8 hours. Due to high FiO2 requirements and poor pulmonary reserve, the patient remained intubated after his bronchoscopy procedure. Over the following 48 hours, FiO2 requirements improved significantly and his repeat chest x-ray showed almost complete resolution of the pulmonary infiltrates. The patient was successfully extubated to 2 liters of oxygen via nasal cannula on the third day. Supplemental oxygen was eventually weaned off to room air. There wasn’t significant desaturation observed with the exercise trial. He was discharged home on a gradually tapering dose of oral steroids over 6 weeks. The patient was later seen at the pulmonary clinic for a follow-up visit. He was doing well and denied any significant respiratory symptoms. A follow-up chest x-ray was within normal limits (Figure 4).

Figure 4. Chest x-ray upon follow-up.
Discussion
Acute eosinophilic pneumonia (AEP) is defined by rapid eosinophilic infiltration of the lung tissue, resulting in impaired gas exchange. Presenting symptoms are nonspecific and may include cough, progressive dyspnea, chest pain, and fever (2). Chest imaging of patients with AEP shows diffuse bilateral parenchymal infiltrates. Diagnosis can be made in the appropriate clinical and radiological context, with BAL showing at least 25% eosinophils on the fluid differential, and with no other identifiable causes (1).
The pathogenesis of AEP is not completely understood; however, it is hypothesized to involve a hypersensitivity reaction in patients with genetic susceptibility (3,4). AEP can be associated with many identifiable causes including cigarette smoke most notably, as well as other inhalants, infections, and medications. Although antibiotics and nonsteroidal anti-inflammatory drugs are among the more common inciting medications, injectable naltrexone has been implicated in several case reports (3,5,6,7).
The clinical presentations of AEP can mimic SARS-CoV-2 pneumonia, community-acquired pneumonia, or ARDS; hence, a high index of clinical suspicion is essential to avoid delay in therapy. A confident diagnosis of AEP can usually be made without a lung biopsy in patients who meet the following criteria (8):
1) acute onset of febrile respiratory manifestations (≤ 1-month duration before consultation).
2) bilateral diffuse opacities on chest radiography.
3) hypoxemia, with PaO2 on room air<60 mm Hg, and/or PaO2/FiO2≤300 mm Hg, and/or oxygen saturation on room air<90%.4) lung eosinophilia, with >25% eosinophils on BAL differential cell count (or eosinophilic pneumonia at lung biopsy).
5) absence of known causes of AEP, including drugs, infections, asthma, or atopic disease.
In our case, the patient has met most of the suggested criteria for diagnosing AEP in addition to the presence of a triggering factor (a clear temporal relationship between the development of symptoms and the recent naltrexone injection). However, we met with a few obstacles before making the diagnosis of AEP. During these unprecedented times, any patient presenting with acute hypoxic respiratory failure, and/or ground-glass opacities (both are classic for SARS-CoV-2 pneumonia as well as AEP) must go through an additional screening process to rule out COVID-19, including contact and airborne infection isolation precautions in addition to the standard precautions and SARS-CoV-2 PCR testing.
On the other hand, several recent reports of AEP presumably triggered by SARS-CoV-2 infection had been described (9-10), which was another factor that contributed to making the diagnosis of AEP more challenging in his case and kept COVID-19 high on the differential diagnosis list. Furthermore, our patient received steroids on the initial presentation which likely affected the accuracy of the total eosinophilic counts in the BAL.
AEP has a higher likelihood than chronic eosinophil pneumonia of presenting with more severe symptoms and has a greater potential of rapid progression to respiratory failure. One review study reported 30-80% of AEP patients required intensive care unit admission and another case review noted 20% of AEP patients required mechanical ventilation (4,11). Treatment includes supportive care, recognition and avoidance of identifiable triggers, and systemic corticosteroids. Most patients rapidly improve with prompt corticosteroid treatment and experience complete recovery (1,3). Relapse of AEP rarely occurs (4).
Numerous conditions can cause pulmonary eosinophilia that needs to be differentiated from AEP. Different classifications have been suggested, but we will list the broad categories and most common etiologies including chronic eosinophilic pneumonia, eosinophilic granulomatosis with polyangiitis (EGPA, previously known as Churg-Strauss), drug and toxin-induced eosinophilic lung disease, helminthic, and fungal infection-related eosinophilic lung diseases, idiopathic hypereosinophilic syndrome, neoplasms, interstitial lung disease, coccidioidomycosis, tuberculosis, and allergic bronchopulmonary aspergillosis.
In addition to AEP, several conditions are associated with elevated BAL eosinophils greater than 25%. These conditions include chronic eosinophilic pneumonia, EGPA, tropical pulmonary eosinophilia. Other conditions causing BAL eosinophilia, but less than 25%, include connective tissue disease, drug-induced pneumonitis, fungal pneumonia, idiopathic pulmonary fibrosis, pulmonary Langerhans cell histiocytosis, sarcoidosis.
Finally, multiple medications are implicated in drug-induced AEP, however, naltrexone is still not well recognized as a potential cause. In a recent retrospective review, naltrexone was not included in the medication list compiled (11).
Conclusion
Injectable naltrexone, a long-acting opioid antagonist, is used for the treatment of opioid and alcohol dependence. Although rare, the use of injectable naltrexone is associated with the potentially fatal side effect of AEP. Since AEP shares many clinical attributes with other causes of acute lung injury, including community-acquired pneumonia and SARS-CoV-2 pneumonia, it can be easily overlooked. Therefore, having an accurate history and an appropriate index of suspicion is important for early detection and proper management (3).
References
- De Giacomi F, Vassallo R, Yi ES, Ryu JH. Acute Eosinophilic Pneumonia. Causes, Diagnosis, and Management. Am J Respir Crit Care Med. 2018 Mar 15;197(6):728-736. [CrossRef] [PubMed]
- Katz U, Shoenfeld Y. Pulmonary eosinophilia. Clin Rev Allergy Immunol. 2008 Jun;34(3):367-71. [CrossRef] [PubMed]
- Mears M, McCoy K, Qiao X. Eosinophilic Pneumonia and Extended-Release Injectable Naltrexone. Chest. 2021;160(4): A1676 [Abstract]. [CrossRef]
- Suzuki Y, Suda T. Eosinophilic pneumonia: A review of the previous literature, causes, diagnosis, and management. Allergol Int. 2019 Oct;68(4):413-419. [CrossRef] [PubMed]
- Horsley R, Wesselius LJ. June 2107 Pulmonary Case of the Month. Southwest J Pulm Crit Care. 2017;14(6):255-61. [CrossRef]
- Esposito A, Lau B. Saved by the BAL: A Case of Acute Eosinophilic Pneumonia After Methyl-Naltrexone Injection. Chest. 2019;156(4):A2210 [Abstract]. [CrossRef]
- Korpole PR, Al-Bacha S, Hamadeh S. A Case for Biopsy: Injectable Naltrexone-Induced Acute Eosinophilic Pneumonia. Cureus. 2020 Sep 3;12(9):e10221. [CrossRef] [PubMed]
- Philit F, Etienne-Mastroïanni B, Parrot A, Guérin C, Robert D, Cordier JF. Idiopathic acute eosinophilic pneumonia: a study of 22 patients. Am J Respir Crit Care Med. 2002 Nov 1;166(9):1235-9. [CrossRef] [PubMed]
- Araújo M, Correia S, Lima AL, Costa M, Neves I. SARS-CoV-2 as a trigger of eosinophilic pneumonia. Pulmonology. 2022 Jan-Feb;28(1):62-64. [CrossRef] [PubMed]
- Murao K, Saito A, Kuronuma K, Fujiya Y, Takahashi S, Chiba H. Acute eosinophilic pneumonia accompanied with COVID-19: a case report. Respirol Case Rep. 2020 Nov 16;8(9):e00683. [CrossRef] [PubMed]
- Bartal C, Sagy I, Barski L. Drug-induced eosinophilic pneumonia: A review of 196 case reports. Medicine (Baltimore). 2018 Jan;97(4):e9688. [CrossRef] [PubMed]
- Salahuddin M, Anjum F, Cherian SV. Pulmonary Eosinophilia. 2021 Dec 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan–. [PubMed]
Cite as: Breuer M, Ali A. Diagnostic Challenges of Acute Eosinophilic Pneumonia Post Naltrexone Injection Presenting During The COVID-19 Pandemic. Southwest J Pulm Crit Care Sleep. 2022;24(2):26-31. doi: https://doi.org/10.13175/swjpccs002-22 PDF