Akshay Warrier
Akshay Sood, MD, MPH
Division of Pulmonary, Critical Care and Sleep Medicine
Department of Internal Medicine
University of New Mexico School of Medicine
Albuquerque, NM USA
Abstract
The COVID-19 pandemic has necessitated the rise of telehealth modalities to relieve the incredible stress the pandemic has placed on the healthcare system. This rise has seen the emergence of new software, applications, and hardware for home-based physiological monitoring, leading to the promise of innovative predictive and therapeutic practices. This article is a literature-based review of the most promising technologies and advances regarding home-based physiological monitoring of patients with COVID-19. We conclude that the applications currently on the market, while helping stem the flow of patients to the hospital during the pandemic, require additional evidence related to improvement in patient outcomes. However, new devices and technology are a promising and successful venture into home-based monitoring with clinical implications reaching far into the future.
Abbreviations
- ARDS: Acute Respiratory Distress Syndrome
- CGM: Continuous Glucose Monitoring
- COVID-19: Coronavirus disease 2019
- EKG: Electrocardiogram
- FDA: Food and Drug Administration
- HIPAA: Health Insurance Portability and Accountability Act
- HR: Heart Rate
- HRV: Heart Rate Variability
- PP: Prone Positioning
- PPE: Personal Protective Equipment
- RHR: Resting Heart Rate
- RIP: Respiratory Inductive Plethysmograph
- SpO2: Peripheral Capillary Oxygen Saturation
Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-COV-2), which causes the novel coronavirus disease 2019 (COVID-19) infection, has been ravaging the globe. The number of infected cases worldwide has risen to 213 million and deaths beyond 4.4 million by August 2021 (1). Furthermore, healthcare workers are at nearly 12 times higher risk of becoming infected than the general community (2), exposing the dire need for a stronger "telemedicine" infrastructure for home-based patient care (1,3,4,5). Such a system not only needs to provide preventative information to users but also allow them to self-diagnose (using home-based testing kits) and self-triage (using real-time algorithms), thus telling patients when to seek emergency care (2). For the less severe cases, the "hospital-at-home" structure can provide acute care at low cost with coordinated telemedicine visits and necessary at-home treatment. For the hospitalized patients, this system allows an earlier discharge to receive post-illness care at home (2). This, in turn, decreases the burden on the hospitals during the pandemic.
Telemedicine, and the technology to support it, has been available for decades but had not become mainstream in the pre-pandemic era due to funding and licensing complications. The technology generally consists of three main functional units: general provision of information, provider-patient synchronous and asynchronous interactions, and remote monitoring (2). Virtual video-chat technologies and basic remote live monitoring algorithms and software were all ready to be used but had not been previously integrated into a fully functioning home-based health care system (5). However, as the pandemic began to spread, the focus on these specific technologies increased and recently have been implemented into several developing home-based systems. Most current remote monitoring programs have a few key features: scaled asynchronous entry, education and information videos/reports, standardized patient reports, real-time monitoring and modifications by a central platform, and enabled patient requests for feedback and assistance (2). "Digital personal protective equipment" or "digital PPE" such as wearable vital monitors, smart applications, and various other forms of medical monitoring have emerged so that COVID-19 monitoring can happen in real-time and assistance or advice can be algorithmically provided to patients.
Evolution of Smart Applications (Apps) for Home-based Monitoring
The foundation for remote monitoring during the pandemic has been provided by novel applications on smartphones (i.e., smart apps) and websites, and other innovative technologies and software hitting the App and Google Play stores, creating a unique opportunity in telemedicine for COVID-19 (7).
1. Application (App) Characteristics
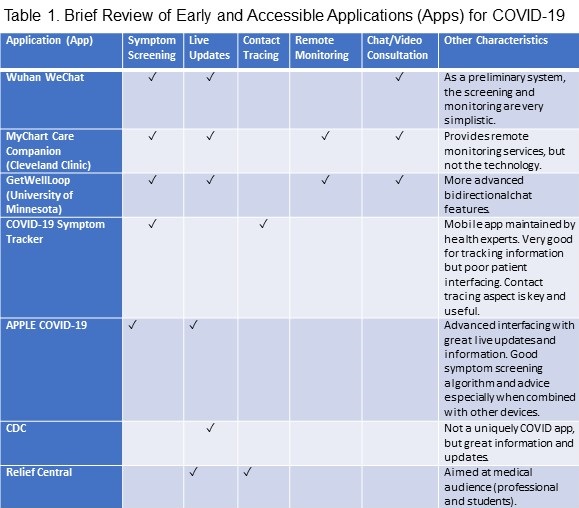
Ideally, a developed application should be able to provide the following services: 1) symptom screening, 2) live updates and information about COVID-19, such as local test availability, 3) contact tracing and mapping of COVID-19 cases, 4) remote monitoring and patient surveillance, and 5) online chat/video consultation with a provider in a secure bidirectional network (6). In addition, the app characteristics should help ensure a streamlined and efficient system using a HIPAA verified data collection service for patients to use and allow big data capabilities for infection epidemiology (7).
2. Current App Developments
One of the earliest apps developed in Wuhan, China, using the popular WeChat platform, established bidirectional communication between a multidisciplinary medical team and quarantined patients through an eCounseling system. Using this app to triage patients, preliminary results show that continuous monitoring of changing symptoms helps in two ways: 1) reduces overcrowding in emergency rooms (ER); and 2) notifies those too afraid to present to the ER if their condition is critical enough to do so (8).
Subsequently, the Cleveland Clinic at Cleveland, USA, put forth an app-based system for real-time monitoring of symptoms, facilitating physician advice and joint decision making, home-based physiological monitoring, and planning for advance directives and related discussions (9). The program used their MyChart Care Companion app, which focused on patient engagement to self-input symptoms and physiological signs (9). Although this app is an excellent first step towards remote patient monitoring, it does not provide patients with technology or equipment for home-based monitoring. Instead, it is an intermediary platform between the provider and the patient.
The GetWellLoop program at the University of Minnesota at Minneapolis, USA, implemented many of the same protocols, such as virtual triaging based on a combination of reported symptoms, conditions, and vital signs, and provision of immediate provider assistance, as needed. In addition, through the use of a smartphone app and basic bidirectional chat software, the program has quickly put in place an adequate but still limited roadmap for patient monitoring (10).
A review of these apps in the context of other more universal apps (Table 1) reveals that despite many desired features in disparate apps, comprehensive software has yet to be developed so far for the general public.
However, the quick implementation of these apps during the pandemic was crucial for stemming the flow of patients into hospitals and in bidirectional home-based disease management in real-time and learning about the emerging disease from the front lines (9). These smart apps will continue to play a significant role in the medical system, greatly assisting, though perhaps not yet replacing, traditional home assessments and telemedicine visits. They offer a window into a secure, well-organized database and communication system as a focal point of remote care to streamline traditional modalities by avoiding significant parts of preliminary assessments and paperwork.
Developments in Home-based Physiological Monitoring
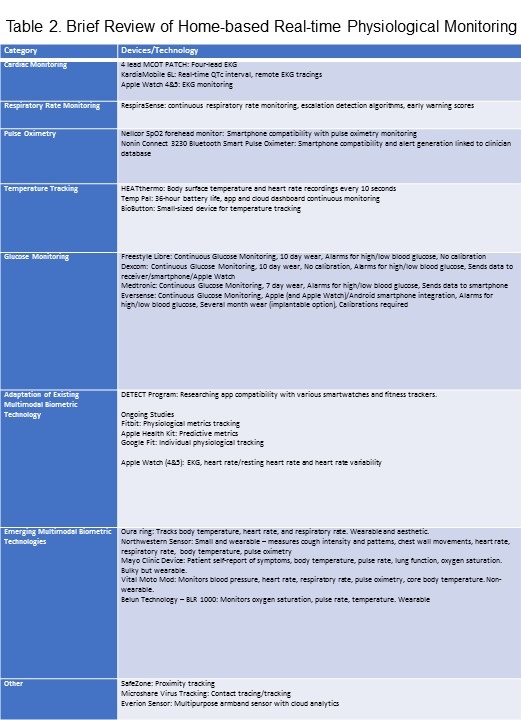
As efforts for vaccination and curative measures continue, research on remote physiologic monitoring has increased (Table 2).
Powerful bioanalytical software coupled with innovative technologies and smart applications offers a pragmatic solution. Realizing the potential of these technologies, the U.S. Food and Drug Administration (FDA) has established a streamlined process for the research and use of home-monitoring devices through various medical platforms (11).
A. Cardiac Monitoring
SARS-COV-2 virus can cause myocarditis, acute coronary syndromes, and arrhythmias, while medications can prolong the corrected QT interval (QTc). Therefore, electrocardiographic (EKG) monitoring, which can help detect tachycardias, conduction defects, and other arrhythmias, and changes of myocardial injury (12), is critical to COVID-19 management (13). Remote single-lead EKG monitoring is considered less accurate than 12-lead telemetry, which is the gold standard. However, several companies now offer mobile solutions for real-time EKG monitoring. After a trial with COVID-19 patients, the FDA cleared one such device, a four-lead MCOT PATCH mobile cardiac telemetry path system for outpatient EKG monitoring (14). Another such device called KardiaMobile 6L by AliveCor offers a real-time QTc measurement service from remote EKG tracings (14). Apple Watches 4 and 5 also have certain EKG monitoring capabilities, modified for diagnostic purposes (15). Beyond EKG monitoring, heart rate (HR), resting heart rate (RHR), and heart rate variability (HRV) biometrics have the greatest predictive capacity (15). These devices illustrate the future of remote monitoring by tracking early heart damage or providing useful warning signs of cardiac status or recovery trajectories (16).
B. Respiratory Rate Monitoring
COVID-19 commonly presents as a lower-respiratory tract infection, necessitating respiratory rate monitoring (17, 18). Due to the relative consistency of an individual's resting respiratory rate, changes can be detected remotely (specifically greater than 27 breaths per minute) (17). Home-based methods for monitoring respiratory rate utilize one of two techniques: 1) respiratory inductive plethysmograph (RIP), which uses belts to measure relative changes in circumference around the abdomen and ribcage, and 2) optoelectronic plethysmography, which uses cameras to map the topography of the torso using local markers. However, new technology has emerged, such as a wearable sensor around the size of a Band-Aid, which remotely monitors local chest wall strain and transmits information to a device through Bluetooth to health care providers (19).
C. Pulse Oximetry
Pulse oximeters, though traditionally used to measure the oxygen saturation of the peripheral blood (SpO2), can also measure heart rate. Monitoring SpO2 is critical to managing the subset of asymptomatic or paucisymptomatic COVID-19 patients with severe hypoxemia (often referred to as "silent hypoxia"). There are generally two categories of pulse oximeters. The traditional method uses light transmission through cutaneous tissue (finger or earlobe). Varying in size, traditional pocket oximeters approved for clinical use can range in cost from 20-50 US dollars. The other major categories of oximeters use reflected light measured by apps that utilize smartphone hardware and software, like the Nellcor SpO2 forehead monitor (20).
An initiative at Cleveland University Hospitals promotes using a disposable wireless finger sensor for home-based SpO2 monitoring (21). Emerging as a costly but highly competitive alternative to others in its field is the Nonin Connect 3230 Bluetooth Smart Pulse Oximeter, which offers smartphone compatibility and alert generation linked with clinician databases, for unexpected SpO2 measurements below 94% (22). Differing branded alternatives have also quickly emerged on the market, providing a cheap and quick reading, albeit with significant and varying inaccuracies, which can be useful in especially urgent contexts.
D. Temperature Tracking
COVID-19 often presents with mild to moderate fever, making body temperature an important metric to track (23). Temperature monitoring has become standard at entry points to buildings to identify and triage those infected (24). In a home-based monitoring setting, fever can be a key warning sign of both the onset of COVID-19 as well as disease trajectory (25). Elevated body temperature is correlated with mortality - the mortality rate being more than 40% higher among those with a maximum body temperature over 40.0° C than those with a lower temperature and increasing for every 0.5° C elevation (26).
There are several modalities for temperature monitoring, the most common of which are electronic thermometers (placed into the mouth, rectum, or armpit); plastic strip surface thermometers which change color to indicate the temperature (limited by their low accuracy); electronic ear thermometers (commonly used but maybe less accurate due to external ear canal blockage); and non-contact forehead infrared thermometers (27). Wearable technology may be effective for frequently measuring and transmitting temperature information. HEATthermo is one such technology that can reliably measure body surface temperature and heart rate every 10 seconds with good reliability (28). The Taiwanese company iWEECARE has come out with the product Temp Pal. The device is the world's smallest thermometer that offers a 36-hour battery life. It sends secure body temperature data to an app and cloud dashboard through Bluetooth for centralized big data tracking (29). These apps and monitoring platforms make it easy for medical professionals to monitor patients and for the latter to seek advice on treatment from the former, using algorithm-based alert messages (30).
E. Glucose Monitoring
Patients with pre-existing diabetes are uniquely vulnerable to SARS-CoV-2 infection and its associated morbidity and mortality. The virus' inflammatory surge (dubbed "cytokine storm") can result in insulin resistance and new-onset diabetes mellitus and its complications. The systemic hyperglycemia can lead to greater viral replication in vivo coupled with a suppressed immune response (31). Continuous glucose monitoring may therefore be helpful in those infected. Recent developments in the field of Continuous Glucose Monitoring (CGM) devices offer a pragmatic solution. Low cost and small wearable devices, like Freestyle Libre, Dexcom, Medtronic, and Eversense, offer a variety of functions, like audio and visual alerts, automatic insulin injections, data confidentiality and integration, strong smartphone and app compatibility, blind data collection for big data studies, and bidirectional clinician interaction (32).
F. Adapting Existing Wearable Biometric Technology
The most logical response to the need for home-based monitoring involves repurposing existing wearable technology to generate useful multimodal biometric data. One-fifth of Americans currently wear some smartwatch or activity tracker, and most of them can give baseline resting heart rate, sleep data, and activity data (33). Duke University investigated the role of an app that tracks smartwatches and fitness trackers in mapping and diagnosing the disease (34,35) through their DETECT program. Recently, new research with larger population input has come to light due to collaborative studies from Stanford, Fitbit, and Scripps, among others, corroborating the use of smartwatches as a predictive tool for disease (15). A recent study of 30,529 people using Fitbit, Apple Health Kit, and Google Fit data showed that individuals' changes in physiological metrics (like HRV, respiratory rate, temperature, oxygen saturation, blood pressure, cardiac output, etc.) tracked by these devices could significantly improve the detection of COVID-19 days before symptoms (33). In a retrospective study sponsored by Stanford University, researchers determined that 63% of COVID-19 cases could have been detected before symptom onset in real-time (36), using smartwatches to generate resting heart rate (RHR) difference data based on standardized values and using anomalies in "heart rate over steps" data (36). Other studies have also bolstered the use of RHR data to detect COVID-19 with smartwatches (37).
G. Emerging Multimodal Biometric Technologies
As the necessity for home-based monitoring grows, wearable multimodal monitoring technologies are being developed. One of the most promising wearable devices is the Oura ring, an aesthetic piece of jewelry that tracks multimodal data. Its use with Smart apps is being investigated (38,39). Northwestern University has invented a wireless sensor, the size of a postage stamp, that rests on the suprasternal notch to monitor cough intensity and patterns, chest wall movements, and vital signs (40,41). Mayo Clinic has started its own project, offering an albeit bulkier device yielding multimodal data, including patient self-reporting of symptoms, lung function (spirometry), and vital signs including oxygen saturation (42,5). Two powerful technology companies, Lenovo and Motorola, have joined efforts to begin certification of their Vital Moto Mod product for multimodal monitoring of vital signs, though not in a continuous or wearable fashion (43). A Chinese company KoKo LLC has agreed to distribute the Belun Technology's system (including the popular Belun ring) for monitoring vital signs. The device, called BLR-1000, uses a SIM (subscriber identification module) card and a HIPAA (Health Insurance Portability and Accountability Act) secured cloud-based system with secure protocols for data transmission to clinicians through a centralized platform (44).
More innovative research is coming in continuous respiratory rate monitoring through the modulation of radio waves and Wi-Fi signals caused by respiration-related thoracic movements, as well as smart garments and mattress pressure sensors (10), combined with cloud-based analytics. Moreover, technologies are being disseminated even as they are developed: Oakland University, California, USA, started handing out skin temperature tracking devices (BioButtons) to its students; employees in Plano, Texas, and football players at the University of Tennessee are already using proximity detectors; Kinexon from Munich is distributing SafeZone proximity trackers to many companies; and GlaxoSmithKline began manufacturing a virus tracking system with Microshare (45).
Although the devices listed above may greatly facilitate home-based physiological monitoring, physical interaction with the provider is still necessary and reassuring for patients. A recent 2020 survey of SWJPCC readership showed that despite the reduced need for documentation, greater overall efficiency, and decreased virus exposure with remote monitoring, patients valued interpersonal interactions associated with physical visits (63). Of course, considerations must be taken into account of those without easy access to technology and the Internet and those requiring additional services such as translation, interpretation, and further testing. Thus, although televisits may have increased out of necessity during the pandemic, they will likely decrease post-pandemic. However, the developed platforms may positively affect harder-to-reach communities if supplemented with the necessary resources, long after the pandemic abates (64).
Promising Home-based Lung Monitoring, Diagnosis, and Treatment Modailities
Lung ultrasound, useful in the point-of-care diagnosis and management of patients with acute respiratory failure, may be helpful in the diagnosis and management of COVID-19 pneumonia (46-49, 62). However, the lack of robust evidence and the need for technology and training renders this option currently not feasible for use in the home setting (62).
Patients at risk for atelectasis use an incentive spirometer to encourage deep, slow breaths (50,51). Although useful for atelectasis, there is little role for incentive spirometry in the treatment of COVID-19. Used in the investigation of asthma, peak expiratory flow rate measures the speed of exhalation (52,53), but its role in the home-based monitoring of COVID-19 is not known. Patients with COVID-19 pneumonia with hypoxia managed at home can be encouraged to use electronically timed treatments of prone-positioning (PP) sessions (54,55).
There still exist other developing investigations into the field of lung testing and early diagnosis. For example, one innovative study delves into machine learning with existing smartphone software and hardware to review breathing sounds. Although not specific to COVID-19 pneumonia, the acoustic technology may help classify subjects with and without pneumonia (56). Another area of investigation is the outpatient use of lung compliance measurements for COVID-19 pneumonia tracking and diagnosis (57, 58). However, the use of lung compliance for this purpose is limited by the normal lung compliance noted in some patients despite severe hypoxemia (58-60).
Conclusion
COVID-19 has radically shifted the healthcare infrastructure; however, depending on how we utilize this system, it may open more doors than close them. The age of telehealth and telemonitoring, and the necessary implications of interactions with the Internet of things, are sure to raise privacy and security questions. Many of the companies and institutions developing smart apps and technologies above prioritize the safety of medical information. From HIPAA-secured clouds to centralized operating databases and governmentally approved/sponsored applications, patients and their security are paramount. A deep and critical analysis of the role that these apps will hold over our healthcare system is not only important but necessary.
The use of remote home-based monitoring to decrease hospital stay is the new future of the medical system. While these technologies are increasing in number and versatility, they are not empirically improving patient outcomes significantly at this time, mainly due to their novelty. The technology’s usefulness and predicted applicability, however, is undeniable in several areas as they become both more intuitive and multifaceted. Using such technological modalities to target rural, underprivileged, and underserved communities could be the stepping-stone to a universal healthcare system. Furthermore, such devices and continuous data streaming to clinician platforms also offer critical benefits to patients with varying conditions outside COVID-19. This system of remote monitoring has changed the healthcare system permanently and will change patient-physician interaction during the pandemic and post-pandemic.
References
- WHO. WHO Coronavirus Disease (COVID-19) Dashboard [Internet]. World Health Organization. World Health Organization; [cited 2021Jan5]. Available from: https://covid19.who.int/
- Marquedant K. Study Reveals the Risk of COVID-19 Infection Among Health Care Workers [Internet]. Massachusetts General Hospital. Massachusetts General Hospital; 2020 [cited 2021Jan5]. Available from: https://www.massgeneral.org/news/coronavirus/study-reveals-risk-of-covid-19-infection-among-health-care-workers
- Sood A, Walker J. The Promise and Challenge of Home Health Services During the COVID-19 Pandemic. Am Fam Physician. 2020 Jul 1;102(1):8-9. [PubMed]
- Gianchandani R, Esfandiari NH, Ang L, Iyengar J, Knotts S, Choksi P, Pop-Busui R. Managing Hyperglycemia in the COVID-19 Inflammatory Storm. Diabetes. 2020 Oct;69(10):2048-2053. [CrossRef] [PubMed]
- Remote Monitoring of COVID-19 Symptoms - About [Internet]. REMOTE MONITORING OF COVID-19 SYMPTOMS. Mayo Clinic; 2020 [cited 2021Jan5]. Available from: https://www.mayo.edu/research/remote-monitoring-covid19-symptoms/about
- Martínez-García M, Bal-Alvarado M, Santos Guerra F, Ares-Rico R, Suárez-Gil R, Rodríguez-Álvarez A, Pérez-López A, Casariego-Vales E; en nombre del Equipo de Seguimiento Compartido TELEA-COVID Lugo; Equipo TELEA COVID-19 (Lugo). Monitoring of COVID-19 patients by telemedicine with telemonitoring. Rev Clin Esp (Barc). 2020 Nov;220(8):472-479. [CrossRef] [PubMed]
- Ming LC, Untong N, Aliudin NA, et al. Mobile Health Apps on COVID-19 Launched in the Early Days of the Pandemic: Content Analysis and Review. JMIR Mhealth Uhealth. 2020 Sep 16;8(9):e19796. [CrossRef] [PubMed]
- Xu H, Huang S, Qiu C, Liu S, Deng J, Jiao B, Tan X, Ai L, Xiao Y, Belliato M, Yan L. Monitoring and Management of Home-Quarantined Patients With COVID-19 Using a WeChat-Based Telemedicine System: Retrospective Cohort Study. J Med Internet Res. 2020 Jul 2;22(7):e19514. [CrossRef] [PubMed]
- Medina M, Babiuch C, Card M, Gavrilescu R, Zafirau W, Boose E, Giuliano K, Kim A, Jones R, Boissy A. Home monitoring for COVID-19. Cleve Clin J Med. 2020 Jun 11. [CrossRef] [PubMed]
- Annis T, Pleasants S, Hultman G, Lindemann E, Thompson JA, Billecke S, Badlani S, Melton GB. Rapid implementation of a COVID-19 remote patient monitoring program. J Am Med Inform Assoc. 2020 Aug 1;27(8):1326-1330. [CrossRef] [PubMed]
- KoKo to distribute Belun's Covid-19 vitals monitoring system in US [Internet]. Medical Device Network. 2020 [cited 2021Jan5]. Available from: https://www.medicaldevice-network.com/news/koko-beluns-covid-19-vitals-monitoring-system-us/
- Cardiac Complications of COVID-19: Signs to Watch for on the ECG [Internet]. GE Healthcare. GE Healthcare; 2020 [cited 2021Jan5]. Available from: https://www.gehealthcare.com/article/cardiac-complications-of-covid-19-signs-to-watch-for-on-the-ecg
- He J, Wu B, Chen Y, Tang J, Liu Q, Zhou S, Chen C, Qin Q, Huang K, Lv J, Chen Y, Peng D. Characteristic Electrocardiographic Manifestations in Patients With COVID-19. Can J Cardiol. 2020 Jun;36(6):966.e1-966.e4. [CrossRef] [PubMed]
- Phend C. Personal ECG Devices Filling in on COVID-19 Drug Monitoring [Internet]. Medical News and Free CME Online. MedpageToday; 2020 [cited 2021Jan5]. Available from: https://www.medpagetoday.com/infectiousdisease/covid19/86107
- Seshadri DR, Davies EV, Harlow ER, Hsu JJ, Knighton SC, Walker TA, et al. Wearable Sensors for COVID-19: A Call to Action to Harness Our Digital Infrastructure for Remote Patient Monitoring and Virtual Assessments [Internet]. Frontiers. Frontiers; 2020 [cited 2021Jan5]. Available from: https://www.frontiersin.org/articles/10.3389/fdgth.2020.00008/full
- Jain S, Workman V, Ganeshan R, Obasare ER, Burr A, DeBiasi RM, Freeman JV, Akar J, Lampert R, Rosenfeld LE. Enhanced electrocardiographic monitoring of patients with Coronavirus Disease 2019. Heart Rhythm. 2020 Sep;17(9):1417-1422. [CrossRef] [PubMed]
- Li J, Fink JB, Ehrmann S. High-flow nasal cannula for COVID-19 patients: low risk of bio-aerosol dispersion. Eur Respir J. 2020 May 14;55(5):2000892. [CrossRef] [PubMed]
- DETECT Health Study. [cited 2021Jan5]. Available from: https://www.detectstudy.org/
- Respiratory Failure [Internet]. PMD Solutions. PMD Solutions; 2020 [cited 2021Jan5]. Available from: https://www.pmd-solutions.com/
- Luks AM, Swenson ER. Pulse Oximetry for Monitoring Patients with COVID-19 at Home. Potential Pitfalls and Practical Guidance. Ann Am Thorac Soc. 2020 Sep;17(9):1040-1046. [CrossRef] [PubMed]
- Michard F, Shelley K, L'Her E. COVID-19: Pulse oximeters in the spotlight. J Clin Monit Comput. 2021 Feb;35(1):11-14. Erratum in: J Clin Monit Comput. 2020 Oct 21. [CrossRef] [PubMed]
- O'Carroll O, MacCann R, O'Reilly A, Dunican EM, Feeney ER, Ryan S, Cotter A, Mallon PW, Keane MP, Butler MW, McCarthy C. Remote monitoring of oxygen saturation in individuals with COVID-19 pneumonia. Eur Respir J. 2020 Aug 13;56(2):2001492. [CrossRef] [PubMed]
- Koh D. Temp Pal smart thermometer helps reduce COVID-19 spread in hospitals [Internet]. MobiHealthNews. HIMSS; 2020 [cited 2021Jan5]. Available from: https://www.mobihealthnews.com/news/asia-pacific/temp-pal-smart-thermometer-helps-reduce-covid-19-spread-hospitals
- Hsiao SH, Chen TC, Chien HC, Yang CJ, Chen YH. Measurement of body temperature to prevent pandemic COVID-19 in hospitals in Taiwan: repeated measurement is necessary. J Hosp Infect. 2020 Jun;105(2):360-361. [CrossRef] [PubMed]
- Tharakan S, Nomoto K, Miyashita S, Ishikawa K. Body temperature correlates with mortality in COVID-19 patients. Crit Care. 2020 Jun 5;24(1):298. [CrossRef] [PubMed]
- Cassoobhoy A. Fever Facts: High Temperature Causes and Treatments [Internet]. WebMD. WebMD; 2020 [cited 2021Jan5]. Available from: https://www.webmd.com/first-aid/fevers-causes-symptoms-treatments
- Tharakan S, Nomoto K, Miyashita S, Ishikawa K. Body temperature correlates with mortality in COVID-19 patients. Crit Care. 2020 Jun 5;24(1):298. [CrossRef] [PubMed]
- Chung YT, Yeh CY, Shu YC, Chuang KT, Chen CC, Kao HY, Ko WC, Chen PL, Ko NY. Continuous temperature monitoring by a wearable device for early detection of febrile events in the SARS-CoV-2 outbreak in Taiwan, 2020. J Microbiol Immunol Infect. 2020 Jun;53(3):503-504. [CrossRef] [PubMed]
- Chung YT, Yeh CY, Shu YC, Chuang KT, Chen CC, Kao HY, Ko WC, Chen PL, Ko NY. Continuous temperature monitoring by a wearable device for early detection of febrile events in the SARS-CoV-2 outbreak in Taiwan, 2020. J Microbiol Immunol Infect. 2020 Jun;53(3):503-504. [CrossRef] [PubMed]
- Cappon G, Vettoretti M, Sparacino G, Facchinetti A. Continuous Glucose Monitoring Sensors for Diabetes Management: A Review of Technologies and Applications. Diabetes Metab J. 2019 Aug;43(4):383-397. [CrossRef] [PubMed]
- Fever and COVID-19 [Internet]. Together. St. Jude's Children Hospital; 2020 [cited 2021Jan5]. Available from: https://together.stjude.org/en-us/care-support/covid-19-resources/fever-and-covid-19.html
- Coronavirus (COVID-19) Update: FDA allows expanded use of devices to monitor patients' vital signs remotely [Internet]. U.S. Food and Drug Administration. FDA; 2020 [cited 2021Jan5]. Available from: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-allows-expanded-use-devices-monitor-patients-vital-signs-remotely
- Phillips N. Peak Expiratory Flow Rate: Purpose, Preparation, and Procedure [Internet]. Healthline. Healthline Media; 2018 [cited 2021Jan5]. Available from: https://www.healthline.com/health/peak-expiratory-flow-rate
- Quer G, Radin JM, Gadaleta M, Baca-Motes K, Ariniello L, Ramos E, Kheterpal V, Topol EJ, Steinhubl SR. Wearable sensor data and self-reported symptoms for COVID-19 detection. Nat Med. 2021 Jan;27(1):73-77. [CrossRef] [PubMed]
- CovIdentify. [cited 2021Jan5]. Available from: https://covidentify.covid19.duke.edu/
- Capodilupo E. Tracking Respiratory Rate and the Coronavirus [Internet]. WHOOP; 2020 [cited 2021Jan6]. Available from: https://www.whoop.com/thelocker/respiratory-rate-tracking-coronavirus/
- Mishra T, Wang M, Metwally AA, et al. Pre-symptomatic detection of COVID-19 from smartwatch data. Nat Biomed Eng. 2020 Dec;4(12):1208-1220. [CrossRef] [PubMed]
- Zhu T, Watkinson P, Clifton DA. Smartwatch data help detect COVID-19. Nat Biomed Eng. 2020 Dec;4(12):1125-1127. [CrossRef] [PubMed]
- Wearable wonderland: how tech is tackling Covid-19 - Medical Technology: Issue 32: October 2020 [Internet]. Wearable wonderland: how tech is tackling Covid-19 - Medical Technology, Issue 32, October 2020 [cited 2021Jan5]. Available from: https://medical-technology.nridigital.com/medical_technology_oct20/wearable_tech_covid-19
- Fowler G. Perspective: Wearable tech can spot coronavirus symptoms before you even realize you're sick [Internet]. The Washington Post. WP Company; 2020 [cited 2021Jan5]. Available from: https://www.washingtonpost.com/technology/2020/05/28/wearable-coronavirus-detect/ Morris A. [Internet]. Monitoring COVID-19 from hospital to home: First wearable device continuously tracks key symptoms. Northwestern University; 2020 [cited 2021Jan5]. Available from: https://news.northwestern.edu/stories/2020/04/monitoring-covid-19-from-hospital-to-home-first-wearable-device-continuously-tracks-key-symptoms/
- Price S. Discover the unique COVID-19 remote monitoring device [Internet]. Health Europa. Health Europa; 2020 [cited 2021Jan5]. Available from: https://www.healtheuropa.eu/discover-the-unique-covid-19-remote-monitoring-device/101138/
- Watson AR, Wah R, Thamman R. The Value of Remote Monitoring for the COVID-19 Pandemic. Telemed J E Health. 2020 Sep;26(9):1110-1112. [CrossRef] [PubMed]
- Temperature measurement: MedlinePlus Medical Encyclopedia [Internet]. MedlinePlus. U.S. National Library of Medicine; 2020 [cited 2021Jan5]. Available from: https://medlineplus.gov/ency/article/003400.htm
- Caretaker Medical: Wireless Vital Sign Monitoring. 2020 [cited 2021Jan5]. Available from: https://www.caretakermedical.net/
- Singer N. The Hot New Covid Tech Is Wearable and Constantly Tracks You [Internet]. The New York Times. The New York Times; 2020 [cited 2021Jan5]. Available from: https://www.nytimes.com/2020/11/15/technology/virus-wearable-tracker-privacy.html
- Mongodi S, Orlando A, Arisi E, et al. Lung Ultrasound in Patients with Acute Respiratory Failure Reduces Conventional Imaging and Health Care Provider Exposure to COVID-19. Ultrasound Med Biol. 2020 Aug;46(8):2090-2093. [CrossRef] [PubMed]
- Tung-Chen Y. Lung ultrasound in the monitoring of COVID-19 infection. Clin Med (Lond). 2020 Jul;20(4):e62-e65. [CrossRef] [PubMed]
- Convissar DL, Gibson LE, Berra L, Bittner EA, Chang MG. Application of Lung Ultrasound During the COVID-19 Pandemic: A Narrative Review. Anesth Analg. 2020 Aug;131(2):345-350. [CrossRef] [PubMed]
- Smith MJ, Hayward SA, Innes SM, Miller ASC. Point-of-care lung ultrasound in patients with COVID-19 - a narrative review. Anaesthesia. 2020 Aug;75(8):1096-1104. [CrossRef] [PubMed]
- Cedars-Sinai Medical Center. Improving Lung Capacity After COVID-19: Cedars-Sinai [Internet]. Cedars. Cedars-Sinai Medical Center; 2020 [cited 2021Jan5]. Available from: https://www.cedars-sinai.org/newsroom/improving-lung-capacity-pre--and-post-covid-19/
- Eltorai AEM, Szabo AL, Antoci V Jr, Ventetuolo CE, Elias JA, Daniels AH, Hess DR. Clinical Effectiveness of Incentive Spirometry for the Prevention of Postoperative Pulmonary Complications. Respir Care. 2018 Mar;63(3):347-352. [CrossRef] [PubMed]
- Ruffin R. Peak expiratory flow (PEF) monitoring. Thorax. 2004 Nov;59(11):913-4. [CrossRef] [PubMed]
- Iwasawa T, Sato M, Yamaya T, Sato Y, Uchida Y, Kitamura H, Hagiwara E, Komatsu S, Utsunomiya D, Ogura T. Ultra-high-resolution computed tomography can demonstrate alveolar collapse in novel coronavirus (COVID-19) pneumonia. Jpn J Radiol. 2020 May;38(5):394-398. [CrossRef] [PubMed] Erratum in: Jpn J Radiol. 2020 Apr 22.
- Pugliese F, Babetto C, Alessandri F, Ranieri VM. Prone Positioning for ARDS: still misunderstood and misused. J Thorac Dis. 2018 Jun;10(Suppl 17):S2079-S2082. [CrossRef] [PubMed]
- Restrepo RD, Wettstein R, Wittnebel L, Tracy M. Incentive spirometry: 2011. Respir Care. 2011 Oct;56(10):1600-4. [CrossRef] [PubMed]
- Faezipour M, Abuzneid A. Smartphone-Based Self-Testing of COVID-19 Using Breathing Sounds. Telemed J E Health. 2020 Oct;26(10):1202-1205. [CrossRef] [PubMed]
- Woods AD. COVID-19 – Not Your "Typical" ARDS [Internet]. COVID-19 – Not Your "Typical" ARDS | Lippincott NursingCenter. Lippincott Nursing Center; 2020 [cited 2021Jan5]. Available from: https://www.nursingcenter.com/ncblog/april-2020/covid-19-not-your-typical-ards
- Komorowski M, Aberegg SK. Using applied lung physiology to understand COVID-19 patterns. Br J Anaesth. 2020 Sep;125(3):250-253. [CrossRef] [PubMed]
- Lucero D 3rd, Mandal S, Karki A. Lung Compliance in a Case Series of Four COVID-19 Patients at a Rural Institution. Cureus. 2020 Jul 30;12(7):e9472. [CrossRef] [PubMed]
- Gargani L, Soliman-Aboumarie H, Volpicelli G, Corradi F, Pastore MC, Cameli M. Why, when, and how to use lung ultrasound during the COVID-19 pandemic: enthusiasm and caution. Eur Heart J Cardiovasc Imaging. 2020 Sep 1;21(9):941-948. [CrossRef] [PubMed]
- Gattinoni L, Chiumello D, Caironi P, Busana M, Romitti F, Brazzi L, Camporota L. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020 Jun;46(6):1099-1102. [CrossRef] [PubMed]
- Luna Gargani, Hatem Soliman-Aboumarie, Giovanni Volpicelli, Francesco Corradi, Maria Concetta Pastore, Matteo Cameli, Why, when, and how to use lung ultrasound during the COVID-19 pandemic: enthusiasm and caution, European Heart Journal - Cardiovascular Imaging, Volume 21, Issue 9, September 2020, Pages 941–948, [CrossRef]
- Robbins RA, Robbins JR. Results of the SWJPCC Telemedicine Questionnaire. Southwest J Pulm Crit Care. 2020;21:66-72. [CrossRef]
- Baum A, Kaboli PJ, Schwartz MD. Reduced In-Person and Increased Telehealth Outpatient Visits During the COVID-19 Pandemic. Ann Intern Med. 2021 Jan;174(1):129-131. [CrossRef] [PubMed]
Disclosures
No disclosures of any personal or financial support or author involvement with organization(s) with financial interest in the subject matter, or any actual or potential conflict of interest.
Cite as: Warrier A, Sood A. Home-Based Physiological Monitoring of Patients with COVID-19. Southwest J Pulm Crit Care. 2021;23(3):76-88. doi: https://doi.org/10.13175/swjpcc005-21 PDF
 Friday, October 15, 2021 at 8:00AM
Friday, October 15, 2021 at 8:00AM