Rodrigo Vazquez, MD1
Cristina Vazquez Guillamet, MD1
Mohamed Adeel Rishi, MD2
Jorge Florindez, MD4
Priya S Dhawan, MD3
Sarah E. Allen, MD1
Constantine A Manthous, MD5
Geoffrey Lighthall MD, PhD6
Affiliations: University of New Mexico School of Medicine1 Albuquerque NM. McNeal Hospital2, Berwyn Il. Mayo Clinic Arizona3, Scottsdale AZ. Bridgeport Hospital4, Bridgeport CT. Yale School of Medicine5, New Haven CT. Stanford University School of Medicine6, Stanford CA.
Study Sites: Stanford University Medical Center, Stanford CA. McNeal Hospital, Berwyn Il and Bridgeport Hospital, Bridgeport CT.
Abstract
Purpose: Technological advances in intensive care unit may lead physicians to question or omit portions of the physical exam. Our goal is to assess the opinions of intensive care unit physicians about physical examination in modern day medicine.
Methods: Subjects included physicians on medical intensive care unit teams at one university hospital and two university-affiliated teaching hospitals. Participants responded to an interview divided into two sections: (1) A semi-structured interview including open-ended questions on the management of four critical care scenarios and on the utility of physical exam; (2) Multiple-choice questions about physical exam.
Main Results: The response rate was 100%. A total of 122 individuals, 16(13%) attendings, 24(20%) fellows and 82(67%) residents, responded. Half 61 (50%) considered physical examination to be of limited utility in the intensive care unit. Fifteen percent of answers to the clinical scenarios were reasoned based on physical examination. Most extended the definition of physical examination to include data derived from monitoring 119(97%), life support 121(99%) and bedside imaging devices 112(92%). Residents 45(37%), students 35(29%) and nurses 35(29%) were recognized as the team members who examine patients the most.
Conclusion: Physical examination was considered useful by half of the physicians. Percussion is the least appreciated component. The role of nurses examining patients is recognized. A new definition of physical examination that extends beyond the patient to include monitoring, life support and bedside imaging is proposed to revitalize bedside clinical medicine.
For accompanying editorial click here.
Introduction
Physical examination is one of the mainstays of clinical activities at the bedside. The many maneuvers and signs of the physical exam were developed and described over the last two centuries when most patients presented in advanced states of disease with obvious physical examination findings (1). In the last few decades, advances in fields of imaging, laboratory and bedside monitoring technologies have increased expectations for early and accurate diagnoses, often before physical exam findings become apparent (2).
In Critical Care Units (ICU) patients have severe presentations of diseases, making it likely to encounter diagnostic physical examination findings; however ICUs have easy access to imaging and automated physiologic measurements that may lead physicians to question, or omit portions, of the physical examination.
In this context, we sought the opinions of physicians working in intensive care units about physical examination in modern day medicine.
Material and Methods
The study was divided into two sections. The first is based on mixed methods analysis (qualitative and quantitative) of semi-structured interviews with open-ended questions. The second is based on the quantitative analysis of multiple-choice questions.
Setting
The study was conducted in 2011, in three ICUs in three states. One of three hospitals (Stanford) was a 32-bed closed medical-surgical unit in a university medical center hospital. The other two hospitals were 16-17 bed closed medical units at university-affiliated community teaching hospitals. All three hospitals had postgraduate residencies in Internal Medicine and Pulmonary and Critical Care Medicine, and were equipped with electronic medical records systems and computer acquisition and storage of bedside data. At the time of the study, Stanford had begun an initiative to increase the use and appreciation of physical examination in its medical school (3). No other confounding variables were apparent. The Investigational Review Boards of each center approved the protocol independently and waived the need for written consent.
Eligible subjects included residents, critical care fellows, and attending physicians on ICU rotations. Investigators approached all candidates for possible participation; subjects were informed that the study would evaluate their approach to diagnosis and treatment of ICU patients but not about its specific focus on physical examination.
Data collection
Demographic data collected included age, gender, type of specialty and subspecialty training, and level of training.
First section
Semi-structured interviews on how four hypothetical ICU clinical vignettes would be managed; questions were chosen by consensus of the authors and responses were open-ended.
Subjects were presented with this introductory statement: “I’m going to present you with four clinical scenarios. I would like you to explain how you would manage these clinical scenarios in real life. This is not an exam. We are just interested in how physicians practice.” The following four case scenarios were then introduced: “You have to manage an Intensive Care Unit patient with: 1) hypoxemia, 2) hypotension, 3) dyspnea and 4) oliguria. What would you do?”
After discussing management of the case scenarios, subjects were then asked: “What’s your opinion about the utility of physical exam in the ICU?”
Second section
Multiple-choice questions were then asked of each subject:
- How frequently do you examine your patients? Answer choices (or options): “Always, sometimes, never”
-Who do you think examines their patients the most? Answer choices: “Attendings, fellows, residents, students or nurses”
-Which data obtained at the bedside should be included in an updated definition of physical exam in the ICU? Inspection? palpation? percussion? auscultation? venous lines? arterial line data? ventilator data?, bedside ultrasonography? Answer options: “Strongly agree, agree, disagree, strongly disagree”
The interview was pilot tested in six subjects to verify subjects had a clear understanding of the questions. Investigators performing the interviews were the same within each center and were trained prior to subject enrollment. Investigators read the questions in the same order. Subsequent questions were not revealed until the previous question had been answered. Responses were recorded and transcribed.
Analysis
Data analysis used a mixed-methods approach for the first section. The transcriptions were analyzed looking for key actions or ideas around which the rest of the response was organized, we called these “codes”. For example if an individual answered: “I would auscultate the lungs, order an arterial blood gas and a chest radiograph ” the codes would be lung auscultation, arterial blood gas and chest radiograph. After analyzing all the answers we decided to group “codes” into categories and report them as percentages of all the answers provided, four clinical scenarios per each of the 122 participants. For the previous example the categories would include: auscultation, laboratory test and radiology.
Mention of the physical exam was categorized as: a) mentions physical examination (e.g. “ I would examine the patient…”), b) mentions physical examination or the intention to go to the bedside, c) describes a reasoned physical examination (e.g.“ I would auscultate the lungs, if I heard wheezes then I would…”).
Illustrative comments were highlighted. The findings of each interviewer were checked against each other. In case of discrepancy, answers were compared to reach a consensus.
The analysis of the second section was quantitative.
We used the statistical package Stata 11. Chi2 was used to compare rates. Factorial logistic regression and logistic regression were used to evaluate categorical and continuous data when appropriate. A p -value of <0.05 was considered significant.
Results
A total of 122 individuals were approached for study participation and all agreed to participate. The subjects included 16 (13%) attendings, 24 (20%) fellows and 82 (67%) residents. The average age was 32 years (range 24-65). There were 79 (65%) males. Most respondents had Internal Medicine training 116 (95%) and had attended medical school in the US 76 (62%).
First section
Clinical scenarios
Categories identified during the responses to the clinical scenarios are summarized in Table 1.

We report mention of the physical exam in three not mutually exclusive categories: a) mentions physical examination (e.g. “ I would examine the patient…”), b) mentions physical examination or the intention to go to the bedside, c) describes a reasoned physical examination (e.g.“ I would auscultate the lungs, if I heard wheezes then I would…”).
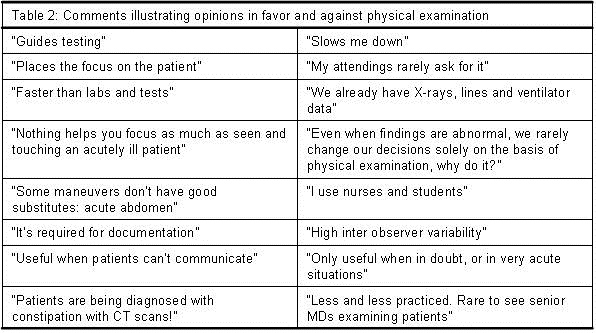
Answers to “What’s your opinion about the utility of physical exam in the ICU?” The physical exam was considered to be of “limited utility” by 61(50%) of the respondents. Table 2 includes answers that illustrate opinions for and against the utility of the physical exam.
According to the respondents, the components of the physical exam that remain useful in the intensive care unit are: a) general appearance; b) the neurological exam; c) abdominal exam since there are no adequate monitoring devices; d) anterior auscultation of the chest to detect pneumothoraces, effusions or cardiac murmurs; and e) examination of the skin.
Second section, multiple-choice questions
A. How frequently do you examine your patients?
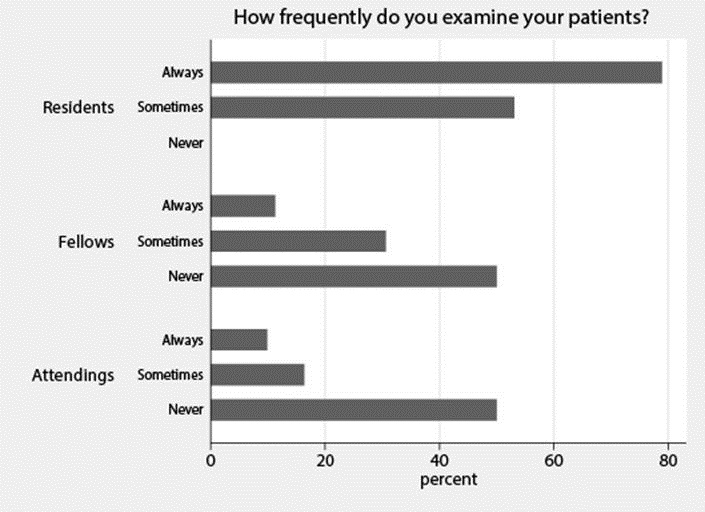
Figure 1. Histogram with the percentages of each of the possible answers to question: How frequently do you examine your patients?
B. Who do you think examines patients the most? (Figure 2).
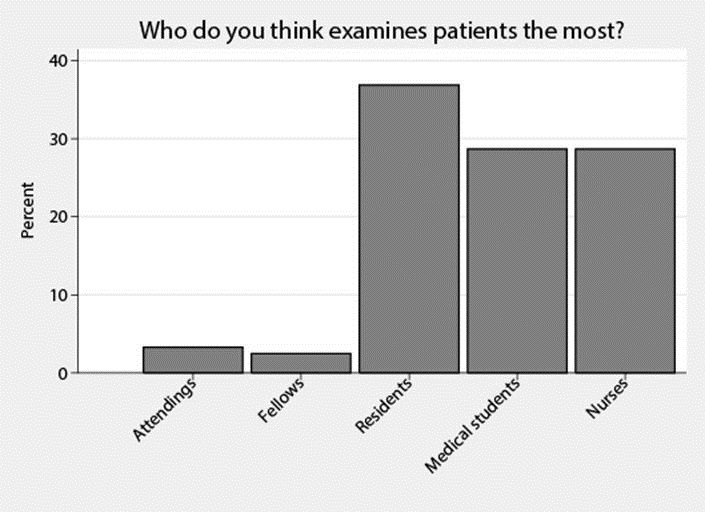
Figure 2. Histogram with the percentages for each of the possible answers to question: Who do you think examines patients the most?
C. Which data obtained at the bedside should be included in an updated definition of physical exam in the ICU?
At least 90% of the respondents agreed to include data obtained through inspection 112 (100%), auscultation 118 (97%), data from ventilators 121 (99 %), arterial lines 120 (98%), central lines 118 (97%), and bedside ultrasound 112(92%). Palpation 107(88%) and percussion 79 (65%) did not exceed the 90% threshold.
Besides the intergroup comparisons noted above, there were no statistically significant differences between responses based on level of training, age, gender, location of medical school training or hospital (p>0.05).
Discussion
We report the opinion of 122 physicians with regard to physical examination in the intensive care unit and the way they reported using the physical exam in four hypothetical clinical scenarios. We found that half of the physicians reported they considered physical exam useful, but only 15 percent mentioned physical exam in deducing answers to the clinical scenarios. Percussion was the least appreciated component of the physical exam. There was generalized agreement that the inclusion of data derived from bedside imaging, monitoring, and life support devices into an updated definition of physical examination would be valuable. Nurses and students were recognized as the team members who examined their patients the most. Study participants provided explanations behind their opinions.
The findings that only 50% of the physicians found the standard physical examination to be useful, and that only 15 % mentioned the physical exam in deducing their case scenario answers suggests a low appreciation for the standard physical exam. Available literature on the utility of the physical exam supports our current study findings. In one study the time spent at the bedside during clinical rounds was down to 11% from an historical 75%, with most of the time spent in hallways or conference rooms (4,5). In an ethnographic study, residents felt it was unnecessary to examine their patients in the ICU as long as they had monitoring and a good nurse (6).
Perceptions from patients and the general public support our findings that use of the physical exam is low. In a questionnaire to ambulatory patients, 56 patients perceived 113 omissions in their physician visit, the most common omissions being those related to what they felt were missed portions of the physical examination (7). Mass media has produced the following headlines: Physician revives a dying art: the physical; Is physical exam facing extinction? and Not on the Doctors’ checklist but touch matters (8-10).
Comments by our study participants help explain these findings. Participants had more confidence in the accuracy of data provided by monitoring devices and imaging than in findings of their physical exams. Some participants mentioned that it was difficult to convince peers to change management based on physical examination findings alone. Participants also reported there was lack of role modeling physicians performing physical exams.
Attending and fellow physicians were perceived as the team members who examine their patients the least; Attending and fellows validated this perception in reporting examining their patients sometimes or never in more than half of the cases.
An alternative explanation for this perception about the senior team members could be related to differences in diagnostic reasoning between trainees and more experienced physicians (11). Students and residents probably relay more in hypothetic deductive reasoning and collect larger amounts of data, including a more detailed physical examination, to reach a diagnosis. As they become more experienced and start working as fellows and attendings the use of short cuts, heuristics, increases and they reach a diagnosis with smaller amounts of data. Time constraints also make them relay in the information relayed by more junior team members.
Whatever the reason for this perception about senior team members may be, it explains, at least in part, the atrophy of physical examination skills during residency training (12), and feeds a downward spiral with graduates that examine their patients less and less.
Residents, nurses and students on the other hand were recognized as the ones who examined their patients the most. This observation expands the role of nurses in the ICU, and if confirmed by others could change the allocation of responsibilities in the multidisciplinary ICU team.
Although until now our discussion portrays the current poor standings of physical examination in the ICU, we also found hope.
Despite the small proportion of participants basing their reasoning on physical examination during the clinical scenarios, most responses included going to the bedside and almost all participants agreed on extending the physical examination beyond the patient to include data derived from monitoring, life support and bedside imaging devices.
Just as Laennec revolutionized bedside diagnosis with the introduction of the stethoscope (13), a new standardized definition of physical examination including these new bedside diagnostic tools, has the potential to greatly enrich clinical medicine and would reflect the practice of modern medicine better (14,15). Critical care medicine is in a privilege position to lead this conceptual change and spread it to other specialties.
Our study has several limitations. First of all, it describes opinions and behaviors in theoretical scenarios and the answers provided may not correlate with true physician practices. The order and choice of the clinical scenarios and questions may have biased the respondents negatively against the physical exam. In support of our results, once the participants learned about the focus of the study one would have expected an attempt to offer better impressions of themselves with an “over reporting” bias towards the physical examination. However, our results pointed in the opposite direction. It does not correlate the use of physical examination to outcomes.
In regards to the composition of the respondents, residents conformed the great majority. Although one could argue that this limits the generalizability of the results, the proportion of residents, fellows and attending physicians mimics that found in the ICU teams at the participating institutions. Our findings may not be generalized to hospitals in areas with limited resources.
Finally its main limitation and at the same time its main virtue is the generation of new questions that will require new studies with direct observation of team practices and their correlation to patient outcomes.
Conclusion
Physical examination was considered useful by half of the physicians. Percussion is the least appreciated component. The role of nurses examining patients is recognized. A new definition of physical examination that extends to include monitoring, life support and bedside imaging is proposed to revitalize bedside clinical medicine.
Conflict of interests: The authors declare that they have no conflict of interest.
References
- Walker HK, Hall WD, Hurst JW, Walker HK. The origins of the history and physical examination 3rd ed. Boston, MA: Butterworths; 1990.
- Berenguer J, Bruguera M, Gervas J, et al. The Physician of the Future. El Metge del Futur. Fundacion Educaion Medica, Barcelona, Spain; 2009. Available at: http://www.econ.upf.edu/~ortun/publicacions/MedicoDelFuturo.pdf (accessed 1/6/15).
- Verghese A, Horwitz RI. In praise of the physical examination. BMJ. 2009;339:b5448. [CrossRef] [PubMed]
- LaCombe MA. On bedside teaching. Ann Intern Med. 1997;126(3):217-20. [CrossRef] [PubMed]
- Miller M, Johnson B, Greene HL, Baier M, Nowlin S. An observational study of attending rounds. J Gen Intern Med. 1992;7(6):646-8. [CrossRef] [PubMed]
- Bosk CL. Forgive and remember: managing medical failure. 2nd ed. Chicago, IL. University of Chicago Press; 2003. [CrossRef]
- Kravitz RL, Callahan EJ. Patients' perceptions of omitted examinations and tests: A qualitative analysis. J Gen Intern Med. 2000;15(1):38-45. [CrossRef] [PubMed]
- Knox R. The Fading Art Of The Physical Exam. National Public Radio. 2010. Available at: http://www.npr.org/player/v2/mediaPlayer.html?action=1&t=1&islist=false&id=129931999&m=129984296 (accessed 1/6/15).
- Ofri D. Not on the doctor's checklist but touch matters. The New York Times. August 2, 2010.
- Grady D. Physician revives a dying art: the physical. The New York Times. October 11, 2010. Available at: http://www.nytimes.com/2010/10/12/health/12profile.html?pagewanted=all&_r=0 (accessed 1/6/15).
- Sackett DL, Tugwell P, Guyatt GH. Clinical epidemiology: a basic science for clinical medicine, 2nd ed. Boston: Little, Brown; 1991.
- Mangione S, Nieman LZ. Cardiac auscultatory skills of internal medicine and family practice trainees. A comparison of diagnostic proficiency. JAMA. 1997;278(9):717-22. [CrossRef] [PubMed]
- Laennec R. Traite de l’auscultation mediate. Bull Acad Natl Med. 1819;151:393-398.
- COBATRICE Domain 2: diagnosis: assessment, investigation, monitoring and data interpretation. Available at: http://www.cobatrice.org/Data/ModuleGestionDeContenu/PagesGenerees/en/02-competencies/0B-diagnosis/8.asp (accessed 1/6/15).
- American Association of Chest Physicians. Critical care ultrasonography. http://www.chestnet.org/Education/Advanced-Clinical-Training/Certificate-of-Completion-Program/Critical-Care-Ultrasonography (accessed 1/6/15).
Reference as: Vazquez R, Vazquez Guillamet C, Adeel Rishi M, Florindez J, Dhawan PS, Allen SE, Manthous CA, Lighthall G. Physical examination in the intensive care unit: opinions of physicians at three teaching hospitals. Southwest J Pulm Crit Care. 2015;10(1):34-43. doi: http://dx.doi.org/10.13175/swjpcc165-14 PDF
 Wednesday, February 11, 2015 at 1:04PM
Wednesday, February 11, 2015 at 1:04PM 








