Rene Franco-Elizondo MD
Soumya Patnaik MD
Kuan-Hsiang Gary Huang MD, PhD
Jorge Mora MD
Albert Einstein Medical Center
Philadelphia, Pennsylvania
Abstract
Imaging studies, such as high resolution computerized tomography (HRCT) and magnetic resonance imaging (MRI) facilitate the evaluation of mediastinal masses. However, the definite characterization of such masses can be ascertained only after tissue sampling is obtained and analyzed. Some mediastinal masses, like bronchogenic cysts, can be misdiagnosed as solid masses or lymphadenopathy in imaging studies, due to the variable densities of the cyst contents. More invasive tests, like fine needle aspiration or surgical resection of the bronchogenic cyst, may be necessary when HRCT fails to provide an initial diagnosis. We describe two such cases seen at our institution that highlight the implications of establishing a diagnosis of bronchogenic cyst with endobronchial ultrasound (EBUS) - trans-bronchial needle aspiration (TBNA) and discuss the possible therapeutic utility of EBUS-TBNA in select patients with bronchogenic cysts.
Abbreviation List
BAL - Bronchoalveolar lavage
CNS - Coagulase-negative Staphylococcus
CT – Computed tomography
EBUS - Endobronchial ultrasound
EUS – Endoscopic ultrasound
FOB - Fiberoptic bronchoscopy
HRCT - High resolution computerized tomography
MRI - Magnetic resonance imaging
RUL – Right upper lobe
TBNA - Trans-bronchial needle aspiration
VATS - Video-assisted thoracoscopic surgery
Introduction
Modern imaging, particularly high-resolution computed tomography (HRCT) and magnetic resonance imaging facilitate the evaluation of mediastinal masses. However, definite characterization is possible only after tissue sampling is obtained, typically through fine-needle aspiration or surgical resection. Herein, we report two cases of patients with mediastinal masses, where HRCT failed to provide a diagnosis. Bronchogenic cysts in both patients were ultimately diagnosed by endobronchial ultrasound (EBUS) and trans-bronchial needle aspiration (TBNA). The implications of establishing a diagnosis of bronchogenic cyst via EBUS-TBNA and therapeutic approaches are discussed.
Case 1
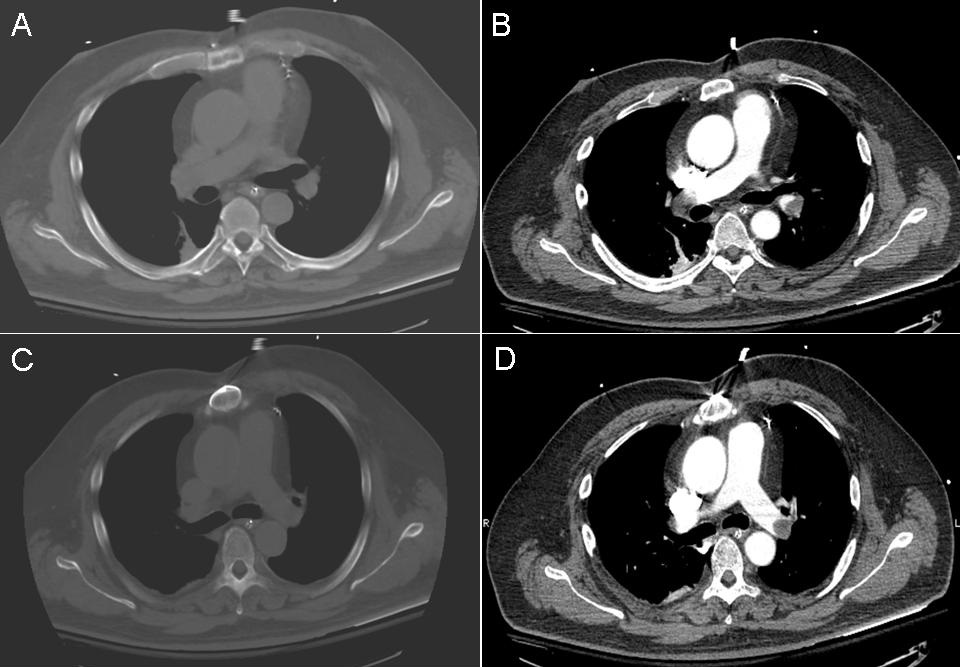
A 68-year-old African American woman with hypertension, diabetes mellitus type 2 and end-stage renal disease, on home hemodialysis, presented to the hospital with central stabbing chest pain, radiating to the back, accompanied by shortness of breath. An initial HRCT chest performed to rule out aortic dissection revealed a large subcarinal mass, measuring 2.3 cm x 6.5 cm x 3.8 cm (AP x transverse x height), that splayed the carina, exerting mass effect on the esophagus, raising suspicion of malignancy (Figure 1).
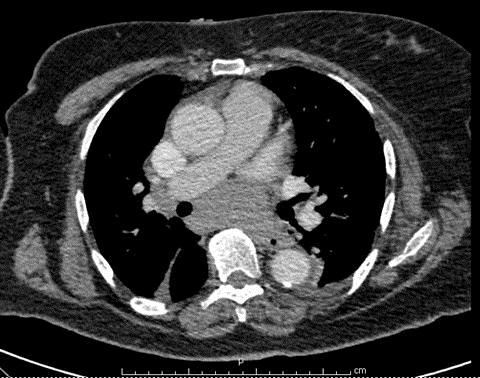
Figure 1. Subcarinal mass. Density ranged from 25-80 Hounsfield Units.
A separate 2.2 cm x 1.7 cm right paratracheal mass, mediastinal lymphadenopathy and many small prevascular lymph nodes were noted. These clinical and imaging findings were concerning for possible lymphoma.
A fiberoptic bronchoscopy (FOB), followed by blind TBNA of the subcarinal space using a Wang needle was attempted. Both, bronchoalveolar lavage (BAL) and TBNA were unrevealing. The patient was found to have persistent coagulase-negative Staphylococcus (CNS) bacteremia, with the first blood cultures being positive at the time of admission. A thorough evaluation, which included an echocardiogram and abdominal HRCT, failed to reveal a source of bacteremia, which was ultimately thought to be related to hemodialysis. Arrangements for outpatient EBUS evaluation of the mediastinal mass and lymphadenopathy were made and the patient was discharged.
A week later, she was readmitted for hypertensive emergency. EBUS was performed during this hospitalization and a cystic mass with heterogeneous-containing material was detected in the subcarinal space (Figure 2).
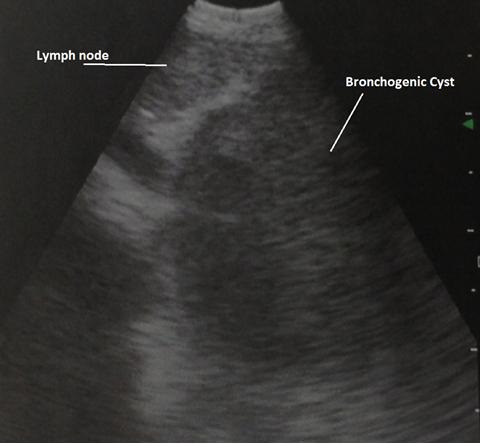
Figure 2 EBUS image of bronchogenic cyst and adjacent subcarinal lymph node.
The needle aspirate was sent for histological analysis and culture, but it was not possible to drain this cystic structure. Cytopathologic analysis showed bronchial and ciliated cells with abundant mucoid material and a diagnosis of bronchogenic cyst was made. Interestingly, the cultures from the aspirated material grew CNS. The patient was discharged with plans for a video-assisted thoracoscopic surgery (VATS) resection of the bronchogenic cyst as an outpatient.
Five days later, the patient was readmitted with symptoms that were concerning for sepsis, and was thus re-started on broad-spectrum antibiotics. She was found to have Enterobacter intermedius bacteremia. She subsequently underwent VATS, with direct aspiration of the bronchogenic cyst. A resection was not performed due to technical difficulties encountered during VATS. Purulent fluid was retrieved from the cyst and Enterobacter intermedius was identified upon analysis and culture of the cyst content. The patient had no further episodes of bacteremia after eight months of follow-up.
Case 2
A 43-year-old woman without significant past medical history was referred to our institute, for evaluation of a pretracheal lymph node seen on a chest HRCT (Figure 3) done for evaluation of new onset dyspnea and wheezing. Upon auscultation, a localized wheeze was noted with deep inspiration in the right upper chest. Her physical exam was otherwise unremarkable.
Figure 3. Chest CT showing subcarinal lymphadenopathy and mass. Density of mass 9-95 Hounsfield units.
A bronchoscopic exam with EBUS evaluation of lymphadenopathy was scheduled. On FOB, the patient was found to have an incidental endobronchial mass occluding the anterior segment of the right upper lobe. EBUS exam revealed an enlarged subcarinal lymph node (8 mm) with an adjacent cystic space containing homogenous hypoechoic material (Figure 4).
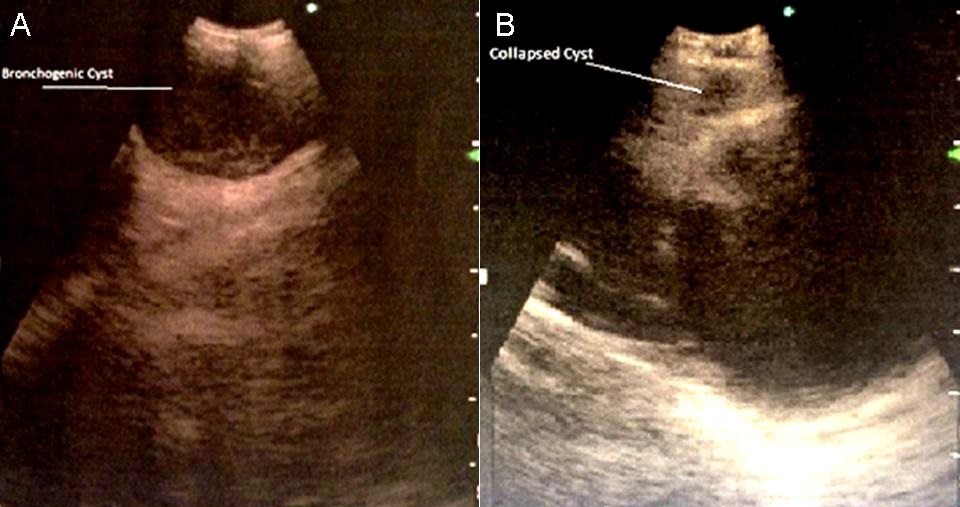
Figure 4. EBUS of bronchogenic cyst. A) Cyst prior to aspiration. B) Collapsed cystic cavity with enlarged lymph node now visible.
Both the lymph node and cystic space were sampled. Ten mL of serous fluid was aspirated from the cystic space, resulting in obliteration of the cavity, as visualized on the ultrasound (Figure 5).
Figure 5.Serous aspirate from cystic cavity.
Full mediastinal staging was done, and only station 11R lymph nodes were found to be enlarged and were sampled. Endobronchial biopsies, brushings and BAL were obtained from RUL endobronchial lesion. The patient was discharged home on empiric antibiotics (amoxicillin/clavulanate) for aspirated bronchogenic cyst. Subsequent fluid analysis revealed abundant macrophages and lymphocytes, consistent with cystic fluid content. Cultures of the fluid were positive for Streptococcus viridians. Lymph node sampling failed to reveal any evidence of malignancy. Interestingly, endobronchial mass biopsies, brushings and fluid cytology also failed to show evidence of malignancy. Only reactive inflammatory cells and benign bronchial elements were detected. The patient was continued on antibiotics for ten days without any evidence of infection.
A repeat bronchoscopy was performed to re-sample the endobronchial lesion. Benign elements were confirmed on the repeat biopsy. Follow up imaging has not been performed to evaluate fluid re-accumulation, since the patient has remained asymptomatic for two months.
Discussion
Mediastinal bronchogenic cysts are congenital anomalies of tracheobronchial origin; they are believed to be a result of an abnormal budding process during the development of foregut. They are often asymptomatic at presentation but can become symptomatic in 30% to 80% of cases due to infection or other complications like compressive efforts (1).
These cysts, being lined by secretary respiratory epithelium, consist of fluid of water density; however, the amount of proteinous mucus and calcium oxalate crystals in them can vary, affecting the imaging features on CT/MRI. A chest CT may reveal spherical masses with water or soft–tissue attenuation. A chest CT may misdiagnose them as soft-tissue masses in about 43% of patients. High attenuation on a chest CT can be a result of calcium oxalate or protein content, or can be due to infection of the cyst content (2, 3).
Due to the variable density in the cyst’s content, bronchogenic cysts can be misdiagnosed as masses or lymphadenopathy on non-invasive testing, as noted in our patients. EBUS can be of great help in diagnosing these lesions. Ultrasound provides an excellent delineation between tissues of different densities, and the absence of flow with color Doppler allows for differentiation from vascular structures. Ultrasonography allows a better delineation of cystic lesions and characterization of their contents (e.g. hypoechoic, isoechoic, heterogeneous, etc.), thereby providing useful diagnostic information. Needle aspiration of cyst contents can bring about not only cytological confirmation of the diagnosis, but also identification of complications such as infected bronchogenic cysts.
Our cases highlight the usefulness of EBUS in the diagnosis of bronchogenic cysts. In the first case, the diagnosis of bronchogenic cyst was made only after EBUS imaging and content aspiration were obtained, despite the initial chest HRCT specifically done to evaluate this mass. In the second case, EBUS imaging established the diagnosis in the absence of any suggestive findings on the HRCT.
The treatment of choice remains the complete surgical resection of the secreting mucosal lining, particularly in complicated cysts (11, 12). However, some authors have reported cases of successful treatment of bronchogenic cysts with EBUS-guided aspiration (4-8). In one case, a patient was followed up for eighteen months without evidence of recurrence (8). The rationale behind this approach is that complete drainage of the cyst obliterates the cyst cavity and prevents further fluid re-accumulation. In our first case, though complete drainage was not achieved with EBUS due to its thick mucoid content, aspiration of the cyst by VATS resulted in resolution without fluid re-accumulation. In our second case, resolution of the cyst was achieved via EBUS-TBNA drainage. These cases underscore the usefulness of aspiration of bronchogenic cysts as an alternative therapeutic approach to surgery in certain scenarios.
Contrary to the above mentioned cases, other case reports have pointed out life-threatening complications after bronchogenic cyst drainage with EBUS-guided FNA, such as pneumonia (9) or purulent pericardial effusion (10). As mentioned elsewhere, empiric antibiotic therapy should be given when a cystic lesion is drained via EBUS-TBNA (13). It should be noted, however, that in some of these case reports, infection post-EBUS-TBNA occurred despite giving empiric antibiotics (9), as in our first case.
The risk of infection should be underscored, as evidenced by the first case; particularly the less frequently reported possibility of bronchogenic cyst infection from bacteremia. The initial EBUS-TBNA cyst aspirate grew CNS, similarly to the blood cultures that were obtained prior to the blind TBNA sample of the mediastinal lesion. This suggests that the contamination of the cyst content could have been due to seeding from CNS bacteremia. However, the final VATS aspirate of the cyst grew Enterecoccus intermedius, which was likely to have been introduced by the EBUS-TBNA at the time of diagnosis. This infection occurred despite the use of antibiotics before and after the procedure. In this regard, the available literature is scant. In a study conducted by Steinfort et al. (14), incidence of bacteremia after EBUS-TBNA was found to be 7%, comparable to reported incidence of bacteremia from regular FOB. It is important to note that although none of these patients experienced clinical signs of infection, none of the biopsies were taken from cystic structures. Data evaluating EBUS-TBNA of mediastinal cystic lesions is conflicting. In a report of 22 patients undergoing EUS-TBNA of suspected mediastinal cyst and receiving periprocedural antibiotics, no infectious complications were found (15). However, several case reports of serious infectious complications after EBUS-TBNA have also been published (16).
Conclusion
Diagnosis of bronchogenic cysts cannot always be made with commonly used chest-imaging modalities such as X-ray or CT. EBUS has proven to be a useful diagnostic tool in the evaluation of some mediastinal masses. Although surgical resection remains the treatment of choice, complete aspiration, by VATS or EBUS, can be a successful therapeutic alternative in patients who are not candidates for surgery. However, the risks should be carefully assessed in each patient, with particular awareness of potential infectious complications. When this approach is taken, empiric antibiotics are recommended.
References
- St-Georges R, Deslauriers J, Duranceau A, Vaillancourt R, Deschamps C, Beauchamp G, Pagé A, Brisson J. Clinical spectrum of bronchogenic cysts of mediastinum and lung in adult. Ann Thorac Surg. 1991;52:6-13. [CrossRef] [PubMed]
- Mc Adams HP, Kirejczyk WM, Rosado-de-Christenson ML, Matsumoto S. Bronchogenic cyst: imaging features with clinical and histopathological correlation. Radiology. 2000;217:441-6. [CrossRef] [PubMed]
- Patel SR, Meeker DP, Biscotti CV, Kirby TJ, Rice TW. Presentation and management of bronchogenic cysts in adult. Chest 1994;106:79-85. [CrossRef] [PubMed]
- Aragaki-Nakahodo AA, Guitron-Roig J, Eschenbacher W, Benzaquen S, Cudzilo C. Endobronchial ultrasound-guided needle aspiration of a bronchogenic cyst to liberate from mechanical ventilation: case report and literature review. J Bronchology Interv Pulmonol. 2013;20(2):152-4. [CrossRef] [PubMed]
- Meseguer SM, Franco-Serrano J. Drainage of a mediastinal cyst by endobronchial ultrasound-guided needle aspiration. Arch Bronconeumol. 2010;46(4):207-8. [CrossRef] [PubMed]
- Dhand S and Krimsky W. Bronchogenic cyst treated by endobronchial ultrasound drainage. Thorax. 2008;63(4):386. [CrossRef] [PubMed]
- Galluccio G, Lucantoni G. Mediastinal bronchogenic cyst's recurrence treated with EBUS-FNA with a long-term follow-up. Eur J Cardiothoracic Surg. 2006; 29(4):627-9. [CrossRef] [PubMed]
- Casal RF, Jimenez CA, Mehran RJ, Eapen GA, Ost D, Sarkiss M, Morice RC. Infected mediastinal bronchogenic cyst successfully treated by endobronchial ultrasound-guided fine-needle aspiration. Ann Thorac Surg. 2010; 90(4):e52-3. [CrossRef] [PubMed]
- Hong G, Song J, Lee KJ, Jeon K, Koh WJ, Suh GY, Chung MP, Kim H, Kwon OJ, Um SW. Bronchogenic cyst rupture and pneumonia after endobronchial ultrasound-guided transbronchial needle aspiration: a case report. Tuberc Respir Dis (Seoul). 2013;74(4):177-80. [CrossRef] [PubMed]
- Gamrekeli A, Kalweit G, Schäfer H, Huwer H. Infection of a Bronchogenic cyst after ultrasonography-guided fine needle aspiration. Ann Thorac Surg. 2013;95(6):2154-5. [CrossRef] [PubMed]
- Cioffi U, Bonavina L, De Simone M, Santambrogio L, Pavoni G, Testori A, Peracchia A. Presentation and surgical management of bronchogenic and esophageal duplication cysts in adults. Chest. 1998;113(6):1492-6. [CrossRef] [PubMed]
- Anantham D, Phua GC, Low SY, Koh MS. Role of endobronchial ultrasound in the diagnosis of bronchogenic cysts. Diagn Ther Endosc. 2011, 2011:468237. [CrossRef] [PubMed]
- Haas AR. Infectious complications from full extension endobronchial ultrasound transbronchial needle aspiration. Eur Respir J. 2009;33(4):935-8. [CrossRef] [PubMed]
- Steinfort DP, Johnson DF, Irving L.B. Incidence of bacteraemia following endobronchial ultrasound-guided transbronchial needle aspiration. Eur Respir J. 2010:36(1):28-32. [CrossRef] [PubMed]
- Fazel A, Moezardalan K, Varadarajulu S, Draganov P, Eloubeidi MA. The utility and the safety of EUS-guided FNA in the evaluation of duplication cysts.GastrointestEndosc. 2005; 62(4):575-80. [CrossRef] [PubMed]
- Jenssen C, Alvarez-Sánchez M. V., Napoléon B and Faiss S. Diagnostic endoscopic ultrasonography: assessment of safety and prevention of complications. World J Gastroenterol. 2012;18(34):4659–76. [CrossRef] [PubMed]
Reference as: Franco-Elizondo R, Patnaik S, Huang K-H G, Mora J. Role of endobronchial ultrasound in the diagnosis and management of bronchogenic cysts: two case descriptions and literature review. Southwest J Pulm Crit Care. 2014;9(2):115-22. doi: http://dx.doi.org/10.13175/swjpcc096-14 PDF
 Wednesday, October 1, 2014 at 8:01AM
Wednesday, October 1, 2014 at 8:01AM