An Observational Study Demonstrating the Efficacy of Interleukin-1 Antagonist (Anakinra) in Critically-ill Patients with Hemophagocytic Lymphohistiocytosis
 Friday, June 28, 2019 at 8:00AM
Friday, June 28, 2019 at 8:00AM Kyle Henry MD, Banner University Medical Center
Robert Raschke MD, University of Arizona College of Medicine-Phoenix
Phoenix, AZ USA
Abstract
Secondary Hhmophagocytic lymphohistiocytosis (HLH) is an underrecognized cause of multisystem organ failure (MSOF) in critically ill adults, associated with high mortality even when recommended etoposide-based treatments are administered. Anakinra, an interleukin-1 receptor antagonist, has shown promise in treating children with HLH. This retrospective case series describes seven adult patients who presented to our ICU with a unremitting syndrome consistent with sepsis / MSOF, who were subsequently diagnosed with secondary HLH and received anakinra. Five of seven (71%) survived. Two non-survivors died secondary to opportunistic fungal infections. Our study contributes to mounting observational evidence regarding anakinra’s possible efficacy in critically ill adults with HLH, and also raises awareness of possible infectious complications of its use.
Introduction
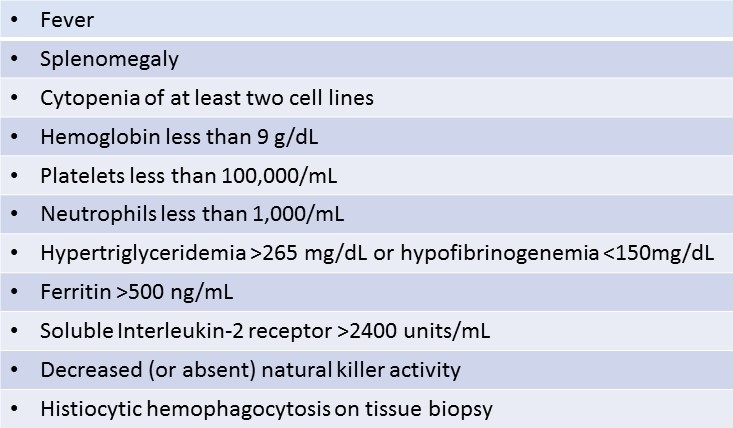
Hemophagocytic lymphohistiocytosis (HLH) is a syndrome characterized by immune dysregulation, hypercytokinemia and tissue infiltration by activated cytotoxic lymphocytes and macrophages (1-3). Primary HLH is a familial syndrome in which gene mutations causing abnormalities of cytotoxic T-lymphocyte and natural killer (NK) cell function result in a systemic hyperinflammatory state. Primary HLH typically presents in the first years of life, progressing to multisystem organ failure (MSOF) and death unless successfully treated with chemotherapy and bone marrow transplantation. Secondary HLH shares clinical features with primary HLH but typically occurs later in life after an underlying illness triggers a dysregulated inflammatory response (3, 4). The diagnosis of primary or secondary HLH is made when five of eight criteria proposed by the International Histiocyte Society are met (Table 1) (5). Heterogeneous groups of patients may satisfy HLH diagnostic criteria, including those in whom HLH is triggered by sepsis, malignancy, and rheumatologic disease (4). Macrophage Activation Syndrome (MAS) is a specific HLH subcategory describing those patients with secondary HLH due to underlying rheumatologic disease (2). The clinical course of secondary HLH is highly variable, progressing relatively slowly in some patients in whom a diagnosis may be made in an outpatient oncology or rheumatology clinic (2, 4, 6-9). Other patients deteriorate rapidly and may require ICU care before the diagnosis of HLH is suspected (3). It has been increasingly recognized that subgroups of patients with HLH have distinctive clinical features, and require special treatment considerations (1, 3-5, 10).
One distinct subgroup consists of adults who present to the intensive care unit (ICU) with sepsis syndrome and MSOF with progressive deterioration despite standard therapy for sepsis (3). Life threatening manifestations in such patients suspected of experiencing HLH may force consideration of presumptive immunotherapy before all HLH diagnostic tests have resulted. Infectious and/or rheumatologic triggers for secondary HLH are eventually found in many, but a clear distinction between sepsis and HLH cannot be made in some (3, 4, 10, 11). The standard treatment protocol for HLH incorporates etoposide – a myelosuppressive chemotherapy agent generally regarded as the standard of care, but has known serious side effects, especially in the setting of hepatic or renal dysfunction typical of sepsis (5, 12). Furthermore, etoposide-based HLH treatment may cause severe immunosuppression leading to opportunistic infections. The mortality of secondary HLH in the adult ICU exceeds 50% (13-16) regardless of the underlying catalyst for the hypercytokinemia. New therapeutic options are desperately needed.
Mounting observational evidence suggests that Anakinra, a recombinant interleukin-1 receptor antagonist (IL-1Ra), may have promise in the treatment of HLH (6,17). Naturally-occurring IL-1Ra is secreted by immune cells to inhibit the pro-inflammatory effects of interleukin 1β (IL-1β) – a key cytokine in the pathogenesis of sepsis and HLH (7, 18). Anakinra was originally developed as a potential therapy for sepsis (7), but is now FDA-approved for use in rheumatoid arthritis. A single case-series describes the successful use of anakinra in critically-ill children with secondary HLH and a few case reports describe its use in critically-ill adults (6, 8, 19, 20). More recently, Wohlfarth and colleagues showed that anakinra is a reasonable option for critically ill patients adults with HLH. At the same time Wohlfarth et al were studying these effects in an Austrian population, we demonstrated similar results in a series of adult patients in the United States who presented to the ICU with sepsis syndrome and underwent treatment with anakinra for secondary HLH.
Methods
This retrospective study was approved by our institutional review board. The setting was the medical and surgical ICU at Banner-University Medical Center Phoenix – a 72-bed ICU in a 650-bed academic tertiary referral center. We identified consecutive adult patients at least 18 years old admitted with sepsis syndrome (known or suspected infection plus acute organ system dysfunction) (21) who subsequently met five or more HLH-2004 diagnostic criteria (Table 1) and received anakinra as part of their treatment regimen between May 2013 and May 2016.
Table 1. HLH-2004 Diagnostic Criteria for Secondary HLH: At least five of eight criteria needed for diagnosis.
Clinical management of patients was not strictly protocolized, but care was provided by an academic 24/7 on-site intensivist service with strong internal consensus regarding the management of HLH. All patients had at least daily complete blood counts and basic metabolic panels. In our practice, diagnostic workup for HLH generally commences upon recognition of unremitting sepsis syndrome with MSOF and bicytopenia. Such patients underwent workup for sepsis and potential causes of secondary HLH that included at minimum: blood cultures, ferritin, fibrinogen, triglycerides, bone marrow aspiration and biopsy, PCR and/or serological testing for systemic lupus erythematosus (SLE), Epstein-Barr virus (EBV), cytomegalovirus, herpes simplex virus, human immunodeficiency virus, hepatitis viruses and coccidioidomycosis (a mycosis endemic in the region). The decision to start HLH therapy was typically based on clinical suspicion plus consistent preliminary laboratory results such as hyperferritinemia, hypofibrinogenemia and/or hypertriglyceridemia, while awaiting the complete results of bone marrow aspiration/biopsy and send-out tests such as soluble interleukin-1 receptor and NK cell functional assays. Presumptive treatment of HLH began with corticosteroids - typically intravenous dexamethasone 10mg/m2 daily. Additional therapies were added at the discretion of the intensivist with consideration of the rapidity of clinical deterioration and likely intolerance of some therapies due to kidney, liver, and/or bone marrow failure. Choice of HLH therapies was based on the HLH-1994 therapy protocol, and influenced by our prior unfavorable experience with etoposide (discussed in conclusions) and recognition of observational literature suggesting that anakinra might be efficacious in patients with secondary HLH. Anakinra was typically given in a dose of 100mg subcutaneously daily, except in patients with creatinine clearance <30ml/min who were dosed every other day.
We retrospectively performed chart reviews to abstract demographics and clinical features related to sepsis and MSOF including infections present on admission, mental status, acute respiratory failure requiring mechanical ventilation, acute renal failure requiring hemodialysis, shock requiring intravenous vasopressors, and liver injury (defined as total bilirubin >2 mg/dL and aminotransferase greater than two times upper limit of normal) (22). The sequential organ failure assessment (SOFA) score was calculated for each patient (21). HLH-2004 diagnostic criteria and the underlying disease process thought to have triggered HLH were abstracted. We documented all treatments including antibiotics and immunosuppressive therapy for HLH, including the dose and duration of anakinra. Outcomes included survival to hospital discharge, duration of fever, mechanical ventilation and renal replacement therapy and ICU length of stay indexed to the time anakinra commenced. Infectious complications occurring during admission after HLH therapy started were also documented. Simple descriptive statistics were performed.
The H Score for each patient was also calculated retrospectively. The H Score is a score used to estimate an individual's risk for having secondary HLH and was recently validated in a 147 patient cohort by Debaugnies et al. (23).
Results
Seven patients were treated with anakinra for a diagnosis of secondary HLH in our ICU between May 2013 and May 2016. Patient ages ranged from 22-59 years – three were female. All patients initially presented to our ICU with a febrile illness consistent with sepsis and received broad-spectrum intravenous antibiotics. Microbiological testing eventually documented infections in two patients – due to influenza A and EBV, respectively. All patients were encephalopathic, five required mechanical ventilation, four required hemodialysis due to acute renal failure, four had liver injury and three required vasopressors due to shock (Table 2).
Table 2. Patient characteristics and some clinical outcomes.

The median SOFA score was 13 (range: 3-17) predicted poor outcome for the group overall (13-16). H Scores for this cohort ranged from 122-263. HLH diagnostic criteria and presumed etiologies are listed in Table 3.
Table 3. Suspected etiology and positive HLH-2004 diagnostic criteria for secondary HLH in patients treated with anakinra: Five of eight criteria required to diagnose HLH.

Two patients were known to have SLE prior to ICU admission and four others were subsequently diagnosed with underlying autoimmune diseases demonstrating a preponderance of MAS in this cohort.
All patients initially received corticosteroids (dexamethasone 10mg/m2 or methylprednisolone >500mg every 12 hours) followed by anakinra. Three patients also received cyclosporine, three underwent plasmapheresis and two received IVIG. Only one patient received etoposide and this was later transitioned to anakinra due to lack of response. Anakinra was started a median of seven days after ICU admission (range 2-58 days). All patients received anakinra 100mg daily, but Q48 hour dosing was used temporarily in five patients who transiently experienced creatinine clearances <30mL/min. Duration of anakinra therapy was 10-159 days - we were unable to determine duration of anakinra after discharge in one patient.
All patients appeared to clinically improve after initiation of anakinra. Of six patients experiencing fever at the time anakinra was started, five defervesced within 24 hours. In five patients that had follow-up ferritin levels within two weeks of starting anakinra, ferritin fell from a median of 7,371 ng/L (range 2,217->40,000) to 4,535 ng/L (range 2,137-26,634). Five of seven patients (71%) survived to hospital discharge with an ICU length of stay (LOS) ranging from 6-17 days and an overall LOS of 17-103 days. Once anakinra was started, liberation from the ventilator occurred within 1-3 days, transfer out of the ICU within 3-5 days, discharge from the hospital within 10-32 days and discontinuation of hemodialysis within 10-44 days.
Death in both non-survivors was due to opportunistic fungal infections - necrotizing pulmonary aspergillosis and disseminated mucormycosis (which occurred despite prophylaxis with amphotericin B). Three other secondary infections all occurred in a single survivor: methicillin-sensitive S. aureus and E coli bacterial ventilator-associated pneumonias and C. difficile colitis all of which responded favorably to treatment while anakinra was continued.
Discussion
Secondary HLH may be more common in the ICU than previously recognized (3), overlapping with and at times indistinguishable from sepsis (3, 4, 10, 11). Rapid clinical deterioration in patients with suspected HLH may force treatment decisions to be made before full diagnostic test results are available. The risk of myelosuppression due to etoposide-based HLH treatment regimens may be intolerable in critically-ill, possibly septic patients with MSOF (4, 12). A therapeutic agent with a more HLH-specific mechanism of action and better safety profile is badly needed.
Anakinra is a recombinant IL-1Ra originally investigated as a potential immune-modulatory treatment for sepsis (7). Phase I and II studies established acceptable safety for further study in sepsis, but a phase III trial failed to demonstrate an overall survival benefit (7). A post-hoc analysis of data from this trial showed that septic patients with hepatobiliary dysfunction, hypofibrinogenemia and thrombocytopenia, such as often seen in secondary HLH, had significantly improved survival if they received anakinra vs placebo (65% vs. 35% 28-day survival, p=0.0007) (7). Anakinra was later approved for use in rheumatoid arthritis (18). Observational studies suggested efficacy in adult onset Still’s disease and systemic juvenile arthritis (9, 24, 25) and in non-critically-ill patients with secondary HLH triggered by these rheumatologic diseases (9, 25, 26). Case reports described the use of anakinra in critically-ill children with secondary HLH (25, 26, 27) and Rajasekaran and colleagues published a case series describing their experience using anakinra in eight critically-ill pediatric patients with secondary HLH/sepsis syndrome (6). All eight patients survived their initial illness, and no infectious complications were attributed to anakinra.
Fourteen cases describing the use of anakinra in adults with secondary HLH have previously been published (6, 8 ,17, 19, 20, 28). Four were due to infections (EBV, CMV, MAC, histoplasmosis, four to autoimmune disease (two with AOSD, SLE antisynthetase syndrome), two post transplantation immunosuppression, one due to acute lymphocytic leukemia and three of unknown trigger. Twelve of 15 (80%) required life support (mechanical ventilation, hemodialysis, vasopressors). All but two received corticosteroids and just over half IVIg. Overall survival was 67%, and no complications of immunosuppression were reported.
The mechanism by which anakinra might ameliorate secondary HLH is not fully elucidated. Secondary HLH (and some forms of sepsis) are characterized by high levels of circulating cytokines including interleukin-6 (IL-6), tumor necrosis factor and interferon-gamma (IFN-γ) (2, 4, 7, 18, 29) - constituting what some have called a “cytokine storm”. Many investigators believe that hypercytokinemia is pathogenic in HLH. Renal failure, cytopenia, coagulopathy and cholestasis have been associated with elevated levels of IL-6 and IFN-γ (2, 18, 28). IL-1β activates lymphocytes responsible for production of these same cytokines (2, 7, 18, 28). IL-1Ra is a competitive inhibitor of IL-1β (7, 29). Therefore IL-1 receptor antagonism by anakinra might inhibit the maladaptive hypercytokinemia characteristic of secondary HLH. This brief explanation oversimplifies a complex and poorly-understood process that requires much further research.
The observed survival rate in our adult patients treated with anakinra (71%) appears favorable compared to that described in other comparable groups of patients (13-16) with survival rates ranging from 25-41%, although we cannot definitively attribute this to treatment effect. It is notable that the majority of our patients had MAS, which has a improved prognosis compared to other forms of HLH when it presents in the outpatient setting, but similar high mortality once the patient develops MSOF and requires intensive care (13-16). This finding supports the concept that secondary HLH of any cause is related to a cytokine storm universal to all underlying catalysts, and that after a critical point the inflammatory cascade becomes increasingly difficult to reverse.
We have previously diagnosed and treated a total of 29 cases of secondary HLH in our ICU. Survival among 22 patients who did not receive anakinra was 14%. This group included eight patients who received etoposide, all of whom died or developed severe neutropenia (WBC <0.5 X 109/L) within a week of its initiation. The patient who survived etoposide did so after her regimen transitioned to anakinra. Statistical comparison of patients in our practice who did or did not receive anakinra was not undertaken due to potential bias and confounding. Controlled prospective trials are required to determine whether anakinra will improve survival of patients with secondary HLH. One such trial is currently under way (ClinicalTrials.gov Identifier: NCT02780583) specifically in regards to MAS.
Two of our patients died from opportunistic fungal infections. Fatal fungal infections have previously been reported to occur in patients receiving treatment for HLH who did not receive anakinra (28) and are likely a result of multiple risk factors including the underlying immune dysregulation associated with HLH, other immunosuppressive therapies and invasive procedures related to ICU care (30, 31), and therefore these infections cannot be specifically attributed to anakinra. We consider prophylactic posaconazole or amphotericin therapy in selected ICU patients at high risk for fungal infections given the evidence of invasive fungal infection prophylaxis including mucormycosis in similarly immunocompromised patients (30, 31).
Conclusions
Our study contributes to mounting observational evidence supporting the hypothesis that anakinra may be efficacious in adult patients presenting to the ICU with life-threatening secondary HLH. In our opinion, it can be considered as first line therapy, in combination with corticosteroids and IVIg, in selected patients for whom renal, hepatic and bone marrow dysfunction put them at higher risk of toxicity due to etoposide. Vigilance is warranted in relation to opportunistic infections, particularly those due to fungi. Prospective controlled trails are needed to definitively establish effective therapy of HLH.
References
- Janka GE, Lehmberg K. Hemophagocytic lymphohistiocytosis:pathogenesis and treatment. Hematology: American Society of Hematology Educational Program 2013:605-11. [CrossRef] [PubMed]
- Schulert GS, Grom AG. Pathogenesis of macrophage activation syndrome and potential for cytokine-directed therapies. Annu Rev Med. 2015:66;145-59. [CrossRef] [PubMed]
- Raschke RA, Garcia-Orr R. Hemophagocytic lymphohistiocytosis:A potentially underrecognized association with systemic inflammatory response syndrome, severe sepsis, and septic shock in adults. Chest. 2011;140:933-8. [CrossRef] [PubMed]
- Castillo L, Carcillo J. Secondary hemophagocytic lymphocytosis and severe sepsis/systemic inflammatory response syndrome/multiorgan dysfunction syndrome/macrophage activation syndrome share common intermediate phenotypes on a spectrum of inflammation. Pediatr Crit Care Med. 2009;10:387-92. [CrossRef] [PubMed]
- Henter J, Horne A, Aricó M, et al. HLH-2004:Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48:124-31. [CrossRef] [PubMed]
- Rajesekaran S, Kruse K, Kovey K, Davis AT, Hassan NE, Ndika AN, Zuiderveen S, Birmingham J. Therapeutic role of anakinra, an interleukin-1 receptor antagonist, in the management of secondary hemophagocytic lymphohistiocytosis/sepsis/multiple organ dysfunction/macrophage activating syndrome in critically ill children. Pediatr Crit Care Med. 2014;15:401-8. [CrossRef] [PubMed]
- Shakoory B, Carcillo JA, Chatham WW, Amdur RL, Zhao H, Dinarello CA, Cron RQ, Opal SM. Interleukin-1 receptor blockade is associated with reduced mortality in sepsis patients with features of macrophage activation syndrome:reanalysis of a prior phase III trial. Crit Care Med. 2016;44:275-81. [CrossRef] [PubMed]
- Loh NK, Lucas M, Fernandez S, Prentice D. Successful treatment of macrophage activation syndrome complicating adult Still's disease with anakinra. Intern Med J. 2012;42:1358-62. [CrossRef] [PubMed]
- Lenert A, Yao Q. Macrophage activation syndrome complicating adult onset Still's disease:a single center case series and comparison with literature. Semin Arthritis Rheum. 2016;45:711-6. [CrossRef] [PubMed]
- Tothova Z, Berliner N. Hemophagocytic syndrome and critical illness: new insights into diagnosis and management. J Intensive Care Medicine. 2014;30:401-12. [CrossRef] [PubMed]
- Stéphan F, Thiolière B, Verdy E, Tulliez M. Role of hemophagocytic histiocytosis in the etiology of thrombocytopenia in patients with sepsis syndrome or septic shock. Clin Inf Dis. 1997;25:1159-64. [CrossRef] [PubMed]
- Donelli MG, Zucchetti M, Munzone E, D'Incalci M, Crosignani A. Pharmacokinetics of anticancer agents in patients with impaired liver function. Eur J Cancer. 1998;34:33-46. [CrossRef] [PubMed]
- Park, HS, Kim DY, Lee JH, et al. Clinical features of adult patients with secondary hemophagocytic lymphohistiocytosis from causes other than lymphoma:an analysis of treatment outcome and prognostic factors. Ann Hematol. 2012;91:897-904. [CrossRef] [PubMed]
- Kaito K, Kobayashi M, Katayama T, et al. Prognostic factors of hemophagocytic syndrome in adults:analysis of 34 cases. Eur J Haematol. 1997;59:247-53. [CrossRef] [PubMed]
- Li J, Wang Q, Zheng W, Ma J, Zhang W, Wang W, Tian X. Hemophagocytic lymphohistiocytosis:clinical analysis of 103 adult patients. Medicine. 2014;93:100-5. [CrossRef] [PubMed]
- Shabbir M, Lucas J, Lazarchick J, Shirai K. Secondary hemophagocytic syndrome in adults:a case series of 18 patients in a single institution and a review of literature. Hematol Oncol. 2011;29:100-6. [CrossRef] [PubMed]
- Wohlfarth P, Agis H, Gualdoni GA, et al. Interleukin I receptor antagonist anakinra, intervenous immunoglobulin, and corticosteroids in the management of critically ill adult patients with hemophagocytic lymphohistiocytosis. J Intensive Care Med. 2017 Jan 1:885066617711386. [CrossRef] [PubMed]
- Gabay C, Lamacchia C, Palmer G. IL-1 pathways in inflammation and human diseases. Nat Rev Rheumatol. 2010;6:232-41. [CrossRef] [PubMed]
- Mehta MV, Manson DK, Horne EM, Haythe J. An atypical presentation of adult-onset Still's disease complicated by pulmonary hypertension and macrophage activation syndrome treated with immunosuppression:a case-based review of the literature. Pulm Circ. 2016;6:136-42. [CrossRef] [PubMed]
- Divithotawela C, Garrett P, Westall G, Bhaskar B, Tol M, Chambers DC. Successful treatment of cytomegalovirus associated hemophagocytic lymphohistiocytosis with the interleukin 1 inhibitor anakinra. Respir Case Rep. 2016;4:4-6. [CrossRef] [PubMed]
- Seymour CW, Liu VX, Iwashyna TJ, et al. Assessment of clinical criteria for sepsis:for the third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315:762-74. [CrossRef] [PubMed]
- Sands KE, Bates DW, Lanken PN, et al. Epidemiology of sepsis syndrome in 8 academic medical centers. JAMA. 1997;278:234-40. [CrossRef] [PubMed]
- Debaugnies F, Mahadeb B, Ferster A, Meuleman N, Rozen L, Demulder A, Corazza F. Performances of the H-Score for diagnosis of hemophagcytic lymphohistiocytosis in adult and pediatric patients. Am J Clin Pathol. 2016;145:862-70.[CrossRef] [PubMed]
- Nigrovic PA, Mannion M, Prince FH, et al. Anakinra as first-line disease-modifying therapy in systemic juvenile idiopathic arthritis:report of forty-six patients from an international multicenter series. Arthritis Rheum. 2011;63:545-55. [CrossRef] [PubMed]
- Bruck N, Suttorp M, Kabus M, Heubner G, Gahr M, Pessler F. Rapid and sustained remission of systemic juvenile idiopathic arthritis-associated macrophage activation syndrome through treatment with anakinra and corticosteroids. J Clin Rheumatol. 2011;17:23-27. [CrossRef] [PubMed]
- Miettunen PM, Narendran A, Jayanthan A, Behrens EM, Cron RQ. Successful treatment of severe paediatric rheumatic disease-associated macrophage activation syndrome with interleukin-1 inhibition following conventional immunosuppressive therapy: case series with 12 patients. Rheumatol. 2011;50:417-9. [CrossRef] [PubMed]
- Kelly A, Ramanan AV. A case of macrophage activation syndrome successfully treated with anakinra. Nat Clin Pract Rheumatol. 2008;4:615-20. [CrossRef] [PubMed]
- Ocon, AJ, Bhatt BD, Miller C and Peredo RA. Safe usage of anakinra and dexamethasone to treat refractory hemophagocytic lymphohistiocytosis secondary to acute disseminated histoplasmosis in a patient with HIV/AIDS. BMJ Case Rep. 2017 Oct 4;2017. pii: bcr-2017-221264. [CrossRef] [PubMed]
- Dinarello CA. Interleukin-1 and its biologically related cytokines. Adv Immunol. 1989;44:153-205. [CrossRef] [PubMed]
- Lawrence TM, Thabet A, Nishino H. Case 10-2011 - a woman with fever, confusion, liver failure, anemia, and thrombocytopenia. N Engl J Med. 2011;364:1259-70. [CrossRef] [PubMed]
- Bajwa SJ, Kulshrestha A. Fungal infections in intensive care unit:challenges in diagnosis and management. Ann Med Health Sci Res. 2013;3:238-44. [CrossRef] [PubMed]
- Cornely OA, Maertens J, Winston DJ, et al. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med. 2007;356:348-59. [CrossRef] [PubMed]
Cite as: Henry K, Raschke RA. An observational study demonstrating the efficacy of interleukin-1 antagonist (anakinra) in critically-ill patients with hemophagocytic lymphohistiocytosis. Southwest J Pulm Crit Care. 2019;18(6):177-86. doi: https://doi.org/10.13175/swjpcc034-19 PDF
Editor's Note: The July 2019 Critical Care Case of the Month is a case presentation of HLH with 0.5 Hour CME credit. Click on the link to be directed to the case.