March 2018 Critical Care Case of the Month
 Friday, March 2, 2018 at 8:00AM
Friday, March 2, 2018 at 8:00AM Babitha Bijin MD
Jonathan Callaway MD
Janet Campion MD
University of Arizona
Department of Medicine
Tucson, AZ USA
Chief Complaints
- Shortness of breath
- Worsening bilateral LE edema
History of Present Illness
A 53-year-old man with history of multiple myeloma and congestive heart failure presented to the emergency department with complaints of worsening shortness of breath and bilateral lower extremity edema for last 24 hours. In the last week, he has had dyspnea at rest as well as a productive cough with yellow sputum. He describes generalized malaise, loss of appetite, possible fever and notes new bilateral pitting edema below his knees. Per patient, he had flu-like symptoms one week ago and was treated empirically with oseltamivir.
Past Medical History
- Multiple myeloma-IgG kappa with calvarial and humeral metastases, ongoing treatment with cyclophosphamide, bortezomib and dexamethasone
- Community acquired pneumonia 2016, treated with oral antibiotics
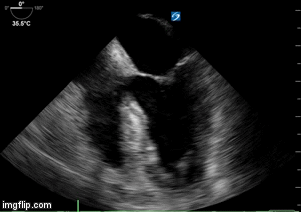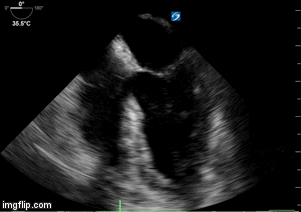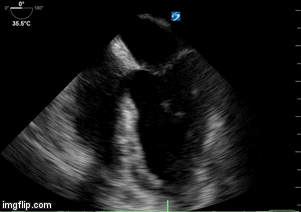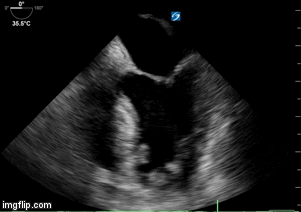
- Heart failure with echo 10/2017 showing moderate concentric left ventricular hypertrophy, left ventricular ejection fraction 63%, borderline left atrial and right atrial dilatation, diastolic dysfunction, right ventricular systolic pressure estimated 25 mm Hg
- Hyperlipidemia
- Chronic kidney disease, stage III
Home Medications: Aspirin 81mg daily, atorvastatin 80mg daily, furosemide 10mg daily, calcium / Vitamin D supplement daily, oxycodone 5mg PRN, chemotherapy as above
Allergies: No known drug allergies
Social History:
- Construction worker, not currently working due to recent myeloma diagnosis
- Smoked one pack per day since age 16, recently quit with 30 pack-year history
- Drinks beer socially on weekends
- Married with 3 children
Family History: Mother with hypertension, uncle with multiple myeloma, daughter with rheumatoid arthritis
Review of Systems: Negative except per HPI
Physical Exam
- Vitals: T 39.3º C, BP 80/52, P121, R16, SpO2 93% on 2L
- General: Alert man, mildly dyspneic with speech
- Mouth: Nonicteric, moist oral mucosa, no oral erythema or exudates
- Neck: No cervical neck LAD but JVP to angle of jaw at 45 degrees
- Lungs: Bibasilar crackles with right basilar rhonchi, no wheezing
- Heart: Regular S1 and S2, tachycardic, no appreciable murmur or right ventricular heave
- Abdomen: Soft, normal active bowel sounds, no tendernesses, no hepatosplenomegaly
- Ext: Pitting edema to knees bilaterally, no cyanosis or clubbing, normal muscle bulk
- Neurologic: No focal abnormalities on neurologic exam
Laboratory Evaluation
- Complete blood count: WBC 15.9 (92% neutrophils), Hgb/Hct 8.8/27.1, Platelets 227
- Electrolytes: Na+ 129, K+ 4.0, Cl- 100, CO2 18, blood urea nitrogen 42, creatinine 1.99 (baseline Cr 1.55)
- Liver: AST 35, ALT 46, total bilirubin1.7, alkaline phosphatase 237, total protein 7.4, albumin 2.
- Others: troponin 0.64, brain naturetic peptide 4569, venous lactate 2.6
Chest X-ray

Figure 1. Admission chest x-ray.
Thoracic CT (2 views)

Figure 2. Representative images from the thoracic CT scan in lung windows.
What is most likely etiology of CXR and thoracic CT findings? (Click on the correct answer to proceed to the second of seven pages)
- Coccidioidomycosis pneumonia
- Pulmonary edema
- Pulmonary embolism with infarcts
- Staphylococcus aureus pneumonia
- Streptococcus pneumoniae infection
Cite as: Bijin B, Callaway J, Campion J. March 2018 critical care case of the month. Southwest J Pulm Crit Care. 2018;16(3):117-25. doi: https://doi.org/10.13175/swjpcc035-18 PDF